Immigration to US Westernizes Asian guts
When immigrants come to the US, it isn't just the people who assimilate. The microbes in their gut also become Westernized after living here. This may predispose them to diseases like obesity.

Have you ever lived long enough in another country to see changes in your overall health? Or perhaps, you have noticed that after a friend moved to the U.S. his health seemed to deteriorate.
Many immigrants arrive in the U.S. healthy. But after living in this country for about a decade, they are at a very high risk of developing obesity. This is not just because these immigrants change their diets or increase caloric intake. Something else is going on. We believe that that part of the problem is a change in the trillions of microscopic creatures that live inside us all – the human microbiome.

In our lab at the University of Minnesota, we study the world of microbes that live in the digestive tract, called the gut microbiome, because these invisible creatures are very important for human health. They help us break down foods that we can’t digest ourselves, help train our immune systems and help us fight off infections. Changes in the gut microbiome are now associated with nearly every major chronic human disease. In fact the data suggest that the microbiome, and changes to it, can cause many of these diseases, including obesity.
Our recent research study, the Immigrant Microbiome Project, explores what happens to people’s gut microbiomes and their health when they move from a developing country to the U.S. We also want to know whether any of these changes may cause obesity.
Gut microbiome diversity falls after move to US
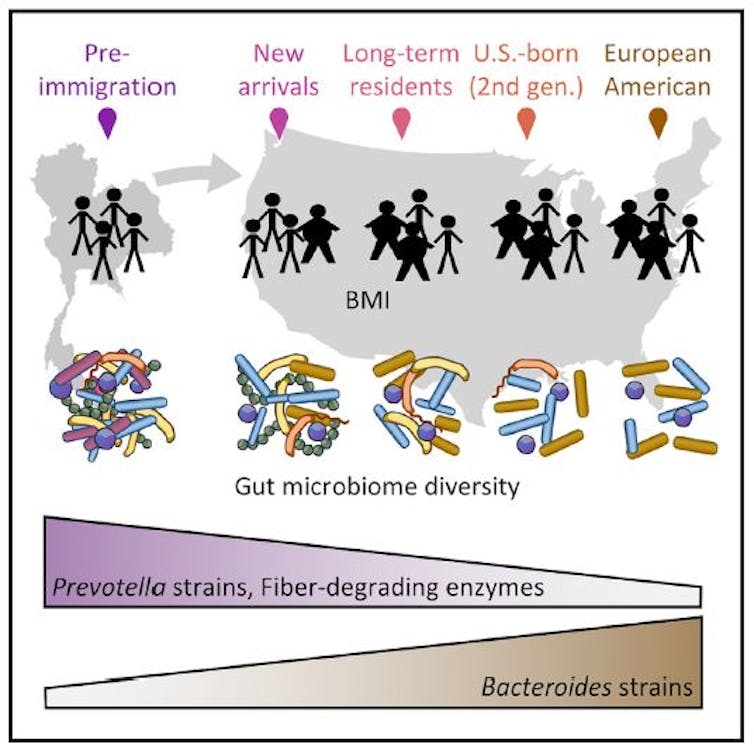
We studied two Asian ethnic groups. One was the Hmong, an ethnic group from the mountainous regions of China, Vietnam, Laos and Thailand. The seond was the Karen, an ethnic group from Myanmar and Thailand. The participants from both of these groups were born and living in Asia, but then moved to the U.S., becoming first-generation immigrants. We also studied second-generation immigrants, those who were born in the U.S. as children of first-generation immigrants.
Having many different species of microbes in the gut is associated with good health. Just as a rainforest with a diverse range of species is more healthy and resilient, a diverse gut microbiome is equipped with a wide variety of tools – genes – to fight against, and recover from, various threats and disturbances. For example, when antibiotics deplete a microbiome, the gut may be colonized by the pathogenic microbe Clostridium difficile.
In our study, we found that the diversity of the gut microbes declined across generations of Hmong and Karen in the U.S. And individuals who were obese had an even greater decline in diversity.
We know from previous studies that in general, obese individuals have a lower microbe diversity in their guts than their lean counterparts. But obese Asians still had higher diversity than Asians who had immigrated to and were now living in the U.S. We also found that the children of immigrants had even fewer species of gut microbes than their parents. This suggests that the modern lifestyle in the U.S. may be causing each generation to lose more of their ancestral microbes.
Gut microbiome Westernized immediately after relocation
In addition to just logging the number of different species, we were also interested in knowing the identity of the different types of bacteria living in the guts of our participants. We were particularly interested in two bacterial groups: Bacteroides, which is commonly found in individuals in Westernized countries, and Prevotella, which is common in individuals in non-Western countries.
These two bacteria are not necessarily good or bad; they’re simply dominant members of the gut microbiomes in different populations around the world. When we examined the gut microbiomes of everyone in our study, we found that, as expected, all of the individuals who were residing in Asia had very high proportions of the non-Western Prevotella. But what we discovered next was surprising.
We discovered that immediately after immigrants moved to the U.S., Bacteroides strains started to replace their native Prevotella strains. After about a decade, first-generation immigrants are no longer dominated by Prevotella, but rather by the U.S.-associated Bacteroides.
Diet explains some changes to the gut microbiome
The obvious explanation for all of these changes is diet, since it is one of the strongest drivers of what species of microbes live in a person’s gut. We found that immigrants who lost Prevotella strains also lost highly specialized enzymes carried by those Prevotella for breaking down certain types of plants. These included palm, coconut, konjac and tamarind, which are commonly eaten in Southeast Asia. It is likely that the immigrants we studied had stopped eating some of these traditional foods after immigration, and the microbes that relied on those plant nutrients failed to grow and multiply and died off.
Although some of the microbes that U.S. immigrants begin to lose seem to be clearly related to changes in diet, we found that many species in the gut microbiome changed much faster and more drastically than their diet changed. We could not explain all of the changes in the gut microbiomes using dietary data alone, suggesting that there are likely other factors that are also affecting the microbiome. These factors could include water sources, antiparasitics or antibiotics, other medications, physical activity, mental health and other environmental exposures.
Although we see that immigration-related microbiome changes are even stronger in obese individuals, we cannot test whether the microbiome is actually causing obesity in our cohort. However, previous studies have shown that having the wrong microbes can cause obesity in mice. It is our hope that we can identify certain dietary interventions that will help U.S. immigrants stay metabolically healthy, or even provide certain microbes that can be used as therapeutics to prevent or treat obesity.
Dan Knights serves as CEO and holds equity in CoreBiome, a company involved in the commercialization of microbiome analysis. The University of Minnesota also has financial interests in CoreBiome under the terms of a license agreement with CoreBiome. These interests have been reviewed and managed by the University of Minnesota in accordance with its Conflict-of-Interest policies. Dan Knights receives funding from the National Institutes of Health and the University of Minnesota Clinical and Translational Science Institute.
Pajau Vangay does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Read These Next
Picky eating starts in the womb – a nutritional neuroscientist explains how to expand your child’s p
While genes do influence some food preferences, positive experiences can help make new tastes easier…
How Homeland Security’s subpoenas and databases of protesters threaten the ‘uninhibited, robust, and
It’s difficult to measure what is lost when an opinion is never voiced and impossible to catalogue…
Supreme Court rules against Trump’s emergency tariffs – but leaves key questions unanswered
The ruling strikes down most of the Trump administration’s current tariffs, with more limited options…





