How does Narcan work? Mapping how it reverses opioid overdose can provide a molecular blueprint for
Naloxone can reverse an overdose in minutes, but exactly how it does this at the molecular level has previously been unclear.
Naloxone, also known by the brand name Narcan, is one of the most important drugs in the United States’ fight against the opioid crisis. It reverses an opioid overdose nearly instantly, restarting breathing in a person who was unresponsive moments before and on the brink of death. To bystanders witnessing it being administered, naloxone can appear almost supernatural.
Although the Food and Drug Administration approved naloxone for medical use in 1971 and for over-the-counter purchase in 2023, exactly how it works is still unclear. Researchers know naloxone acts on opioid receptors, a family of proteins responsible for the body’s response to pain. When opioids such as morphine and fentanyl bind to these receptors, they produce not only pain relief and euphoria but also dangerous side effects. Naloxone competes with opioids for access to these receptors, preventing the drugs from triggering effects in the body. How it does this at the molecular level, however, has been an ongoing question.
In our recently published research in the journal Nature, my team and I were able to provide some definitive evidence of how naloxone works by capturing images of it in action for the first time.
Biology of opioids
To better grasp how naloxone works, it’s helpful to first zoom in on the biology behind opioids.
One member of the family of opioid receptors, MOR – short for µ-opioid receptor – is a central player in regulating the body’s response to pain. It sits on the surface of neurons, mostly in the brain and spinal cord, and acts as a communication hub.
When an opioid – such as an endorphin, the body’s natural painkillers – interacts with MOR, it changes the structure of the receptor. This change in shape allows what’s called a G protein to bind to the receptor and trigger a signal to the rest of the body to reduce pain, induce pleasure, or – in the case of overdose – dangerously slow breathing and heart rate.

In everyday terms, MOR is like a lock on the outside of the cell. The G protein is the mechanism inside the lock that turns when the correct key – in this case, an endorphin or a drug like fentanyl – goes in. For decades, scientists believed that an opioid’s ability to enable this signaling cascade was linked to how effectively it reshaped the structure of the receptor – essentially, whether the lock could open wide enough for the internal mechanism of the G-protein to engage.
Yet, recent research – including our work – has revealed that the critical step to how opioids work is not how wide they open the lock but how well the mechanism works. G proteins act like a switch, releasing one molecule in exchange for another molecule that triggers the protein to send the signal that sets off opioid effects.
In essence, drugs like fentanyl, by acting on the receptor, transmit physical changes to G proteins that result in the switch flipping more rapidly. What we now see is that naloxone jams the mechanism, preventing the switch from flipping and sending the signal.
Capturing the switch
Researchers know that the effects of opioids are triggered when the G protein switch is flipped. But what does this process look like?
For years, attempts to visualize this mechanism were largely limited to two states – before the G protein binds to the µ-opioid receptor, and after the molecule was released from the G protein. The states in between were considered too unstable to isolate. My team and I wanted to capture these unseen states moment by moment as the switch flips and the molecule is released.
To do this, we used a technique called cryo-electron microscopy, which freezes molecules in motion to visualize them at near-atomic resolution. For both naloxone and the opioid drug loperamide (Imodium), we trapped the G protein bound to the opioid receptor right before it released the molecule.
We captured four distinct structural states leading up to the release of the molecule from the G protein.
The first of these, which we call the latent state, is the earliest form of the opioid receptor and G protein after they make contact. We found that both the opioid receptor and the G protein are inactive at this point. Moreover, naloxone stabilizes this latent state. What this means is that naloxone effectively jams the mechanism right at the start, preventing all subsequent steps required for activation.
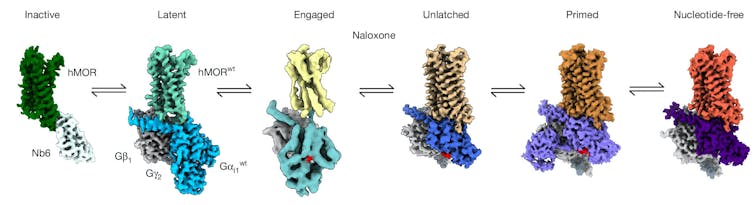
In the absence of naloxone, an opioid drug promotes a transition to the remaining three states: The G-protein rotates and aligns itself with the receptor (engaged), swings open the door blocking the molecule that would trigger the switch from flipping (unlatched), and holds that door open so the molecule can be released (primed) and send the signal to carry out the drug’s effects.
To confirm that our snapshots reflect what’s really happening, we performed extensive computational simulations to watch these four states change over time. Together, these findings point to the molecular root of naloxone’s therapeutic effects: By stalling the opioid receptor and G protein at a latent state, it shuts down opioid signaling, reversing an opioid overdose within minutes.
Visualizing new drugs
Designing a new key for a lock is most successfully done when you know exactly what that lock looks like. By mapping the exact sequence of how opioids interact with opioid receptors and pinpointing where different drugs can intervene in this process, our findings provide a blueprint for engineering the next generation of opioid medicines and overdose antidotes.
For example, one of the persistent challenges with naloxone is that it must often be administered repeatedly during an overdose. This is especially the case for fentanyl overdoses, where the opioid can outcompete or outlast the effects of the treatment.
Knowing that naloxone works by stalling the µ-opioid receptor in an early, latent state suggests that molecules that can bind more tightly or more selectively to this form of the receptor could be more effective at stabilizing this inactive state and thus preventing an opioid’s effects.
By uncovering the structure of molecules involved in opioid signaling, researchers may be able to develop drugs that provide longer-lasting protection against overdose.
This research was supported by the National Institutes of Health.
Read These Next
Fewer new moms are dying in Colorado – naloxone might be one reason why
The opioid reversal drug is distributed directly to new moms at many of Colorado’s birthing hospitals.
How natural hydrogen, hiding deep in the Earth, could serve as a new energy source
Hydrogen demand around the world is projected to grow significantly by 2050. Some of that supply could…
The apocrypha, Christianity’s ‘hidden’ texts, may not be in the Bible – but they have shaped traditi
‘Apocrypha’ means ‘hidden’ in Greek, but it is often used to describe texts that are outside…