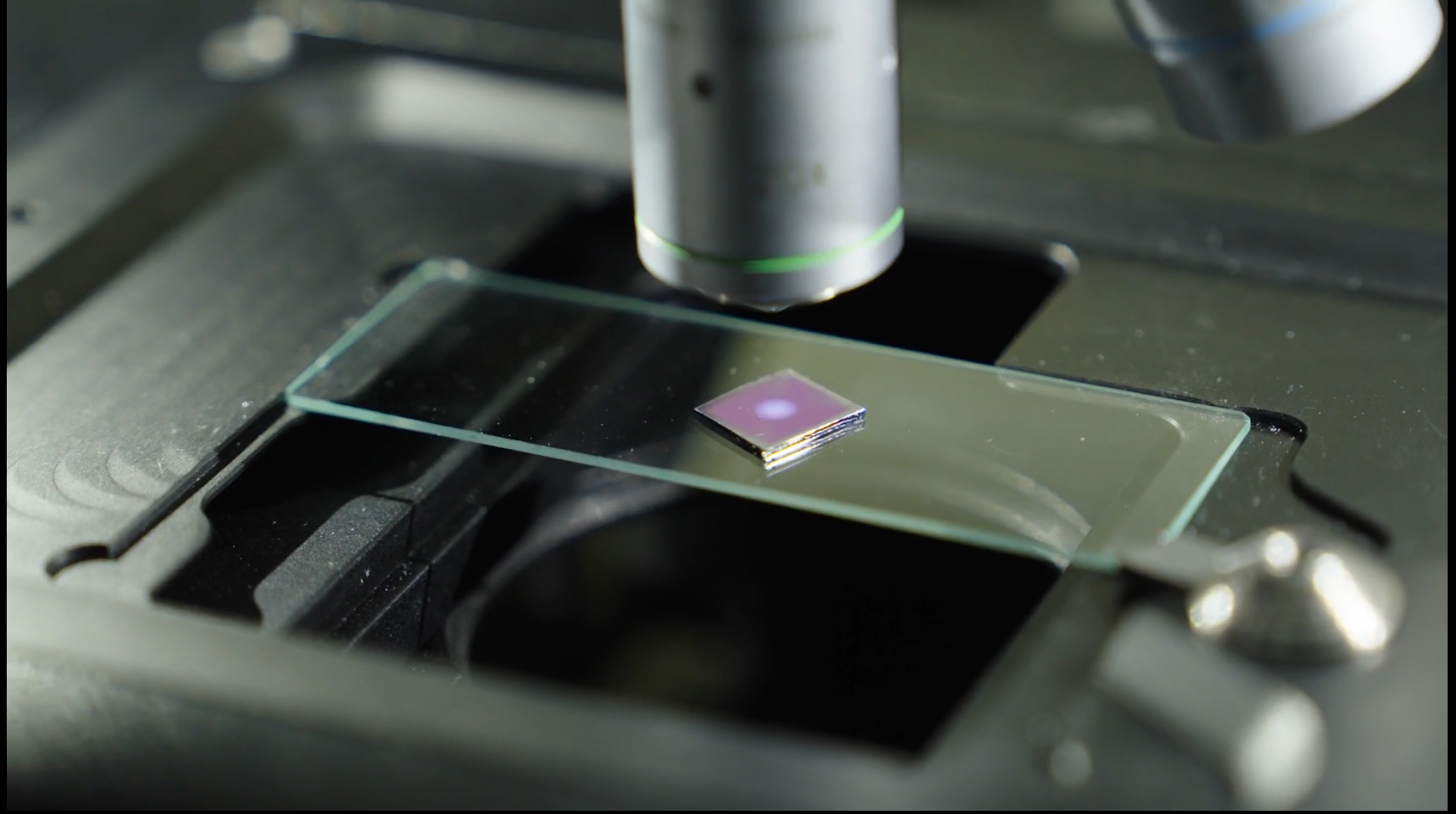
Our team of physicists inadvertently generated the shortest X-ray pulses ever observed
Super short X-ray pulses help scientists study materials at the atomic level. Researchers found that certain lasing effects can make these pulses even shorter.

X-ray beams aren’t used just by doctors to see inside your body and tell whether you have a broken bone. More powerful beams made up of very short flashes of X-rays can help scientists peer into the structure of individual atoms and molecules and differentiate types of elements.
But getting an X-ray laser beam that delivers super short flashes to capture the fastest processes in nature isn’t easy – it’s a whole science in itself.
Radio waves, microwaves, the visible light you can see, ultraviolet light and X-rays are all exactly the same phenomenon: electromagnetic waves of energy moving through space. What differentiates them is their wavelength. Waves in the X-ray range have short wavelengths, while radio waves and microwaves are much longer. Different wavelengths of light are useful for different things – X-rays help doctors take snapshots of your body, while microwaves can heat up your lunch.
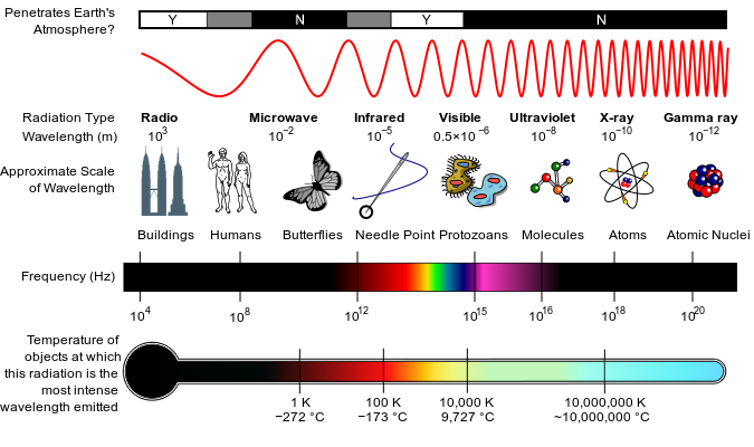
Optical lasers are devices that emit parallel, or collimated, beams of light. They send out a beam where all the waves have the same wavelength – the red light you get from a laser pointer is one example – and oscillate in synchronicity.
Over the past 15 years, scientists have built X-ray free-electron lasers, which instead of emitting beams of visible light emit X-rays. They are housed in large facilities where electrons travel through a long accelerator – depending on the facility, between a few hundred meters and 1,700 yards – and after passing through a series of thousands of magnets they generate extremely short and powerful X-ray pulses.

The pulses are used kind of like flash photography, where the flash – the X-ray pulse – is short enough to capture the fast movement of an object. Researchers have used them as cameras to study how atoms and molecules move and change inside materials or cells.
But while these X-ray free-electron laser pulses are very short and powerful, they’re not the shortest pulses that scientists can make with lasers. By using more advanced technology and taking advantage of the properties some materials have, researchers can create even shorter pulses: in the attosecond region.
One attosecond is one-billionth of a billionth of a second. An attosecond is to one second about what one second is to the 14 billion-year age of the universe. The fastest processes in atoms and molecules happen at the attosecond scale: For instance, it takes electrons attoseconds to move around inside a molecule.
We’re physicists who work with X-ray free-electron lasers. We study what happens when we put different types of materials in the X-ray free-electron pulses’ path. In a new experiment we put copper and manganese samples in the path of highly focused X-ray free-electron laser pulses. We knew the interactions between these elements and the X-ray free-electron laser pulses would generate new X-ray laser pulses.
Originally, we wanted to find out how different chemical forms of the element manganese – for example, manganese-II and manganese-VII – would create small changes in the wavelengths of these newly generated X-ray laser pulses.
But along the way, we found some unexpected results that made the newly generated X-ray laser pulses act strangely. At first, we did not understand why, but when we eventually figured it out, we realized that we had discovered two unique laser phenomena, and that these effects had helped us generate X-ray laser pulses that where much shorter than we’d expected – shorter than the fastest X-ray pulses ever previously generated.
Filamentation – irregular spurts
We found that our new X-ray laser pulses weren’t always shooting out in the forward direction, as we expected. When we increased the intensity of the X-ray free-electron laser pulses, the resulting new X-ray laser pulses spurted out irregularly, in slightly different directions.
For optical lasers, these irregular spurts – or filamentation – result from the index of refraction changing in the laser material. But we didn’t expect to see this effect for X-rays, since materials – including the manganese and copper we used – don’t refract X-rays very much.
However, the high intensity X-ray free-electron laser pulses we used generated the fluctuations at the quantum level in our materials that led to these irregular spurts.
Rabi cycling – a broad spectrum of light
Even more surprising than the filamentation effects we saw was the fact that the X-ray pulses we generated contained a variety of different wavelengths that were more spread out than what we expected to see with the materials we used.
Seventy years ago – five years before the first optical laser was built – physicists Stanley Autler and Charles Townes discovered a strange phenomenon in microwaves known as Rabi cycling. And the spread of wavelengths we saw looked just like Rabi cycling.
Autler and Townes knew that when light hit an atom, the atom would absorb its energy by exciting an electron from one energy level to a higher one. The hole left by that missing electron is filled by an electron that’s coming down from a higher energy level in the atom and releasing – or emitting – this energy difference as light.
What Autler and Townes found was that when the microwaves are very intense, the strong electric field can split each of these energy levels into two distinct levels, called doublets, which have slightly different energies.
These doublets are separated by an energy, or a frequency, known as the Rabi frequency. The Rabi frequency depends on the intensity of the new light. The stronger it is, the larger is the energy separation.
In Autler and Townes’ discovery of Rabi cycling, they used microwaves. The energy splitting was so small that the Rabi frequency was very low, at radio wave frequencies.
In this new study we used X-rays, which have 100 million times shorter wavelengths than microwaves and 100 million times more energy. This meant the resulting new X-ray laser pulses were split into different X-ray wavelengths corresponding to Rabi frequencies in the extreme ultraviolet region. Ultraviolet light has a frequency 100 million times higher than radio waves.
This Rabi cycling effect allowed us to generate the shortest high-energy X-ray pulses to date, clocking in at 60-100 attoseconds.
Future directions and applications
While the pulses that X-ray free-electron lasers currently generate allow researchers to observe atomic bonds forming, rearranging and breaking, they are not fast enough to look inside the electron cloud that generates such bonds. Using these new attosecond X-ray laser pulses could allow scientists to study the fastest processes in materials at the atomic-length scale and to discern different elements.
In the future, we also hope to use much shorter X-ray free-electron laser pulses to better generate these attosecond X-ray pulses. We are even hoping to generate pulses below 60 attoseconds by using heavier materials with shorter lifespans, such as tungsten or hafnium. These new X-ray pulses are fast enough to eventually enable scientists to answer questions such as how exactly an electron cloud moves around and what a chemical bond actually is.
Uwe Bergmann receives funding from the Department of Energy and has previously worked at SLAC and Lawrence Berkeley National Laboratory.
Thomas Linker receives funding from the Department of Energy and works at SLAC National Accelerator Laboratory.
Read These Next
Cuba’s speedboat shootout recalls long history of exile groups engaged in covert ops aimed at regime
From the 1960s onward, dissident Cubans in exile have sought to undermine the government in Havana −…
Nanoparticles and artificial intelligence can help researchers detect pollutants in water, soil and
Tiny particles bounce light around in a unique way, a property that researchers are using to detect…
Tiny recording backpacks reveal bats’ surprising hunting strategy
By listening in on their nightly hunts, scientists discovered that small, fringe-lipped bats are unexpectedly…