Why are elements like radium dangerous? A chemist explains radioactivity and its health effects
Unstable atoms emit fast-moving particles that can damage cells in the human body. Some atoms are far more unstable than others.

Curious Kids is a series for children of all ages. If you have a question you’d like an expert to answer, send it to CuriousKidsUS@theconversation.com.
“What is radium and why is it dangerous?” – Aurora, 10, Laredo, Texas
The element radium can be found in extremely tiny amounts in the Earth’s crust and oceans, and in its pure form it is a soft silvery metal. To an untrained eye, a small piece of radium may look like a chip off a regular gray rock. But radium can invisibly emit radiation – energy and small fragments of itself – that you can’t feel, see or smell. And that invisible radiation can hurt you, without you even noticing right away.
What’s going on with this silent threat that can stealthily damage your body in ways that can take years to reveal themselves?
As a chemist, I’m interested in what makes different elements safe to handle or hazardous. This dangerous release of radiation is called radioactivity, and even though its source may look unassuming, it can burn you or even give you diseases that don’t manifest for years.
Atoms and isotopes
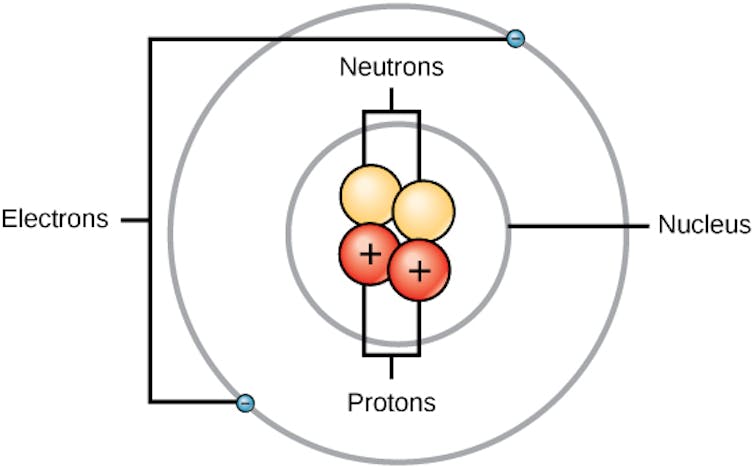
Everything you see around you – your skin, rocks, the pages of books – is all made up of different combinations of extremely small particles called atoms.
An atom has a small, dense center called the nucleus. Negatively charged particles called electrons move around the nucleus. Inside the nucleus, there are two types of particles: positively charged protons and neutral neutrons.
All atoms with the same number of protons in their nuclei are the same element. Besides radium, some elements you may have heard of are carbon and oxygen. All carbon atoms have six protons and all oxygen atoms have eight protons. Radium atoms are much heavier – all radium atoms have 88 protons.
Interestingly, it is possible for atoms of the same element to have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. For instance, two carbon atoms would each have six protons, but one might have six neutrons while another could have seven or eight.
The number of protons and neutrons packed together in the nucleus determines whether the nucleus of an isotope is stable or not. If the nucleus is not stable, problems can arise.
Radioactive decay
The nucleus of each atom wants to be stable, but only certain arrangements of protons and neutrons make that possible. The number of protons and neutrons do not have to be equal, but some combinations make for a happy, or stable, coexistence in the nucleus while others don’t.
A nucleus with an unhappy mix of protons and neutrons might break down or deteriorate in some way. That process is called radioactivity or radioactive decay.
That radioactive decay process releases some form of radiation from the nucleus. This radiation can take the form of tiny particles moving rapidly or high-energy electromagnetic waves emerging from the nucleus. It is that radiation – the high-energy particles and waves shooting out from the nucleus of unstable atomic nuclei – that can make you sick.
There are different types of radioactive decay. In one case, an atom decays by kicking out a small fragment of itself that is made up of two protons and two neutrons. Since the number of protons determines what element we have, decay that changes the number of protons in an atom turns it into a different element.
Radioactive decay can be quite slow, though. It can take thousands of years for one element to decay into a different one.
The case of radium
All radium atoms are unstable and radioactive. Many of these isotopes decay very quickly, but Ra-226, which has 138 neutrons and 88 protons and is the most common, decays the slowest. It takes 1,600 years for half a sample of Ra-226 to decay.
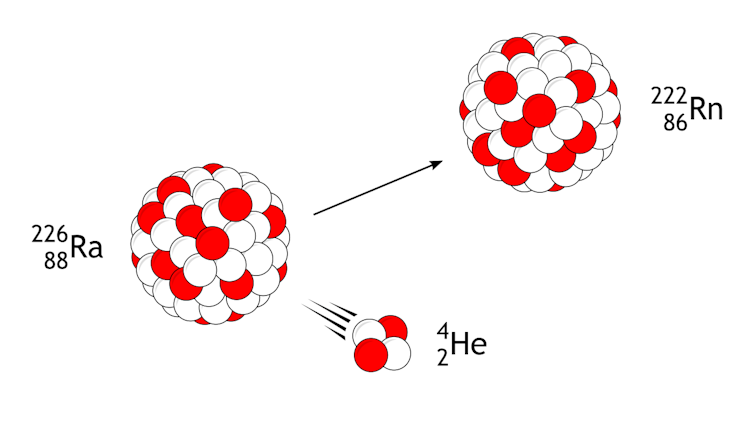
As Ra-226 decays, it loses two protons and two neutrons, which turns it into an isotope of radon. Then the radon decays, and the atom eventually reaches a stable form as the element lead. Each step in that decay series releases more nuclear radiation.
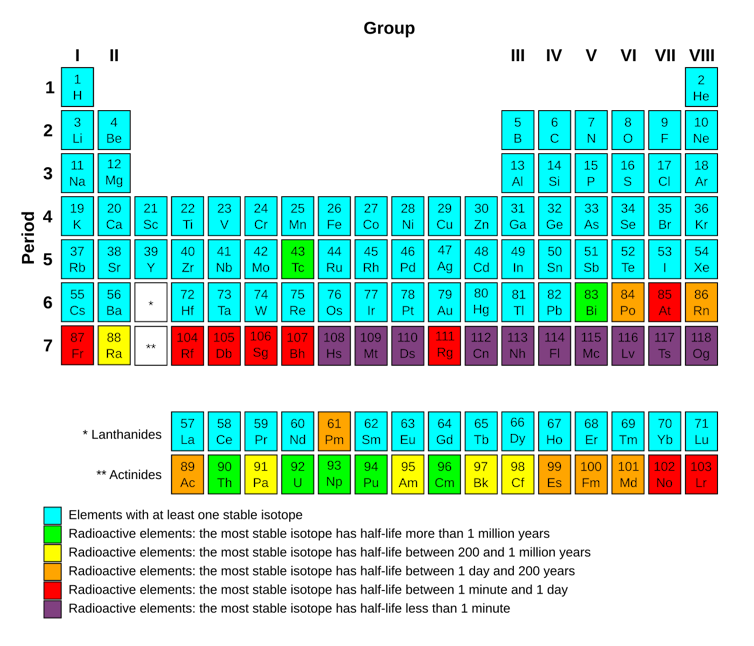
Some other elements in nature with no stable isotope are technetium, polonium, actinium and uranium.
Effects on the human body
The nuclear radiation emitted when radium and other elements decay can damage the cells in the human body. It can lead to cancers or other health problems.

Whether you’re exposed to a lot of radiation quickly, like making the mistake of walking around for a few hours with radioactive material in your pocket, or you’re exposed to just a little over a long time, the high-energy particles and electromagnetic waves from nuclear radiation can lead to serious health problems, including burns and cancers.
Remarkably, even though radioactivity is a threat to life, scientists can control and use it to diagnose and treat diseases – including cancers. If the radiation is delivered precisely to where cancer cells are, the radiation can destroy those rogue cells wreaking havoc in the body.
People who work professionally with radioactive materials need to follow strict guidelines and procedures to protect themselves. They use special shields and radiation detectors, and they minimize the amount of time they’re exposed to any radioactivity.
Pierre and Marie Curie, who discovered radium in 1898, suffered some of the negative effects of radioactivity. Pierre experienced radioactive burns, and Marie died from a blood disease likely caused by chronic radiation exposure. Over 100 years later, her notebooks are still radioactive.
Hello, curious kids! Do you have a question you’d like an expert to answer? Ask an adult to send your question to CuriousKidsUS@theconversation.com. Please tell us your name, age and the city where you live.
And since curiosity has no age limit – adults, let us know what you’re wondering, too. We won’t be able to answer every question, but we will do our best.
Kelling Donald does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Read These Next
Failure of US-Iran talks was all-too predictable – but Trump could still have stuck with diplomacy o
Silence from the US side after a third round of indirect talks and frustration expressed by President…
Kansas revoked transgender people’s IDs overnight – researchers anticipate cascading health and soci
With invalid driver’s licenses and birth certificates, transgender people are at risk for more than…
Iran will respond to US-Israeli strikes as existential threats to the regime – because they are
The latest attack on Iran goes far beyond previous operations by Israel and the US in both scale and…





