Iron nanoparticles can help treat contaminated water – our team of scientists created them out of ex
Researchers compared the environmental footprints of several techniques used to make magnetic nanoparticles.

Today, approximately 1,800,000 acres of land in the United States is used for landfill waste disposal. In terms of volume, the U.S alone generated over 290 million tons of solid waste in 2018, an amount equivalent to about 235,000 Olympic-size swimming pools, assuming an average solid waste density of a half ton per cubic meter.
Roughly 9% – about 26 million tons – of this waste is made up of iron and steel. These are resources with a stable market value used in various civil infrastructure projects. As a team of environmental engineers, we wanted to know whether we could use iron-rich waste to produce iron oxide nanoparticles – a useful tool for combating water pollution and building engineering hardware.
All about nanoparticles
Iron oxide nanoparticles consist of iron and oxygen atoms and, because of their size, they exhibit unique physical and chemical properties. They are extremely small, typically at the nanoscale – one-billionth of a meter – in diameter.
The iron oxide nanoparticles we synthesized were a distinctive group called magnetite and maghemite. Initial studies have shown that nanoparticles in this group could help drugs get to the right part of the body, make batteries in electric vehicles more efficient and improve sensors for detecting toxic gas, as well as sound and motion.
Because these nanoparticles are made of iron, they’re both magnetic and stable. Their tiny size gives them a large surface area relative to their volume, allowing them to grab pollutants in water. Additionally, their magnetic nature makes them ideal for building extremely small and thin electrical components.
In our work, we wanted to find a new way to produce them using waste materials. In our newest study, published in the RSC Sustainability journal, we developed an eco-friendly method to synthesize iron oxide nanoparticles from expired over-the-counter iron supplements. This approach not only gives value to discarded products but also supports a more sustainable and circular method of production.
The research process
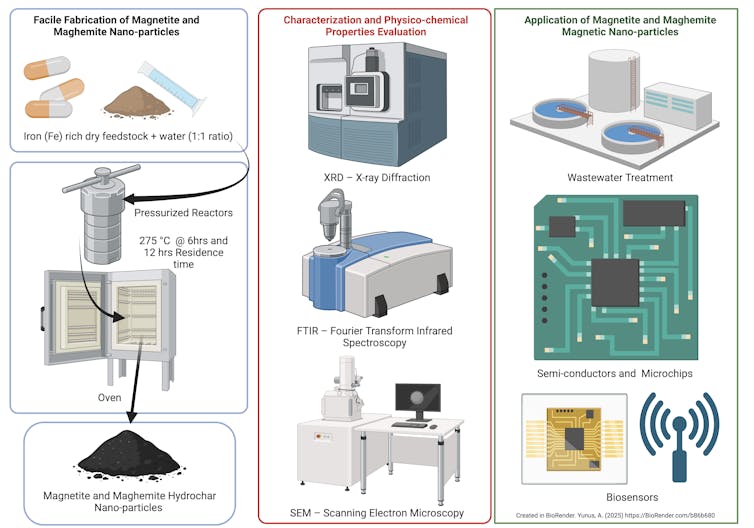
To conduct our study, we used a method called hydrothermal carbonization to produce these magnetic nanoparticles. We were able to source a large amount of expired iron supplements from a local health care center.
The hydrothermal carbonization process uses a turbocharged version of the kind of pressure cooker you might have in your kitchen. For our recipe, we combined 20 grams each of expired iron supplements and water in a specialized pressure reactor. We then cooked the mixture at 527 degrees Fahrenheit (275 degrees Celsius) for six to 12 hours. Under this intense temperature and pressure, the supplements broke down, which produced tiny – 10- to 11-nanometer – particles.
The end product included a solid charcoal-like material called hydrochar, which made up about 20% to 22% of the product. The hydrochar consisted of the iron oxide nanoparticles and graphite, a carbon-rich material that gave the hydrochar its charcoal-like look. The rest became gas and a dark, tarlike liquid separate from the hydrochar.
Hydrothermal carbonization is not the only method used to make iron oxide nanoparticles. There are other conventional methods such as coprecipitation, which involves mixing chemicals to form solids. Another method is pyrolysis, where materials are heated in the absence of oxygen. And finally, gasification, which heats materials in the presence of oxygen.
These methods usually require a higher energy input, around 1,292 to 1,832 degrees Fahrenheit (700 to 1,000 C), or harsh salt chemicals. In contrast, hydrothermal carbonization, the method we used, is water-based and can happen at a low temperature.
We compared our hydrothermal carbonization process’s energy use with other methods and found it had the lowest environmental impact.
From polluted water to clean
The iron oxide nanoparticles we created are very useful for water treatment. They are particularly good at removing oil and heavy metals such as lead, cadmium, zinc and chromium from water. These are pollutants known to cause serious health issues, including cancer.
You can either mix them with polluted water or allow the water to pass through them, similar to a common household filter.
To test their performance, we mixed our iron oxide nanoparticles in wastewater samples containing methylene blue dye, a common pollutant in textile and manufacturing wastewater. We found they removed over 95% of the dye, and because the particles are magnetic, we could remove them from the treated water using a magnet so they didn’t contaminate the water.
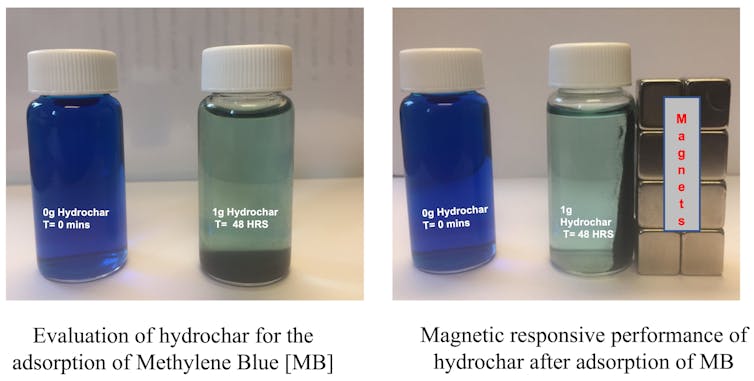
Depending on the type of pollutants in the water, iron oxide nanoparticles can sometimes be reused after they’re heated again.
Moving forward
We produced a small amount of these nanoparticles in the lab for this study. However, large quantities of iron waste are sent to landfills. These include materials such as steel sludge and metal scraps. So in theory, many more of these nanoparticles could be produced in the future. If produced in large enough quantities, large water and wastewater plant filtration systems could use these particles to treat much larger amounts of water.
But landfill waste isn’t all one type of waste. Iron-rich waste may be contaminated with other materials, making its sourcing, sorting and recycling both resource-intensive and costly. To scale up this technology sustainably, researchers will need to first overcome these challenges.
On the bright side, economists predict that alternative metals, including iron oxide nanoparticles, may help meet production demands for future technologies and artificial intelligence. These nanoparticles can be used to manufacture high-performance computing components. These components include magnetic memory storage and semiconductors found in our everyday technologies.
Lots of the critical metals currently used are expensive, scarce or geopolitically sensitive: cobalt, nickel and lithium. As a result, our team is starting to explore how this hydrothermal carbonization-based method can be scaled and applied to other types of waste materials.
Our long-term goal is to expand the tool kit for sustainable nanoparticle production while continuing to address both environmental challenges and materials demands for future innovations.
Ahmed Ibrahim Yunus receives funding from Georgia Tech Renewable Bioproduct Institute and the United States Department of Energy. This research project was headed by Dr. Samuel Darko while supported by Dr. Yongsheng Chen and Dr. Joe F. Bozeman III.
Joe Frank Bozeman III receives funding from the Georgia Institute of Technology's Renewable Bioproduct Institute.
Read These Next
AI’s growing appetite for power is putting Pennsylvania’s aging electricity grid to the test
As AI data centers are added to Pennsylvania’s existing infrastructure, they bring the promise of…
The cost of casting animals as heroes and villains in conservation science
New research shows how these storytelling choices can distort science – and how to move beyond them.
Detroit was once home to 18 Black-led hospitals – here’s how to understand their rise and fall
In the early 20th century, Detroit’s Black medical professionals created a network of health care…





