Mitochondria can sense bacteria and trigger your immune system to trap them – revealing new ways to
Not only do mitochondria serve as the engine of the cell – they also act as watchtowers for the immune system.
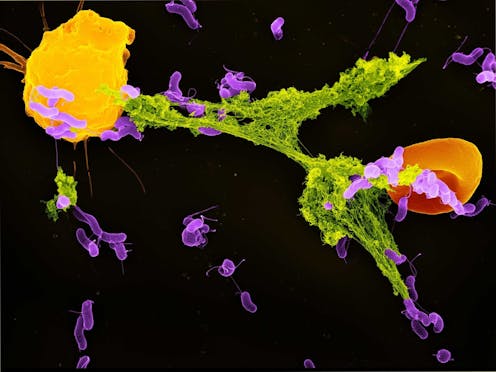
Mitochondria have primarily been known as the energy-producing components of cells. But scientists are increasingly discovering that these small organelles do much more than just power cells. They are also involved in immune functions such as controlling inflammation, regulating cell death and responding to infections.
Research from my colleagues and I revealed that mitochondria play another key role in your immune response: sensing bacterial activity and helping neutrophils, a type of white blood cell, trap and kill them.
For the past 16 years, my research has focused on understanding the decisions immune cells make during infection and how the breakdown of these decision-making processes cause disease. My lab’s recent findings shed light on why people with autoimmune diseases such as lupus may struggle to fight infections, revealing a potential link between dysfunctional mitochondria and weakened immune defenses.
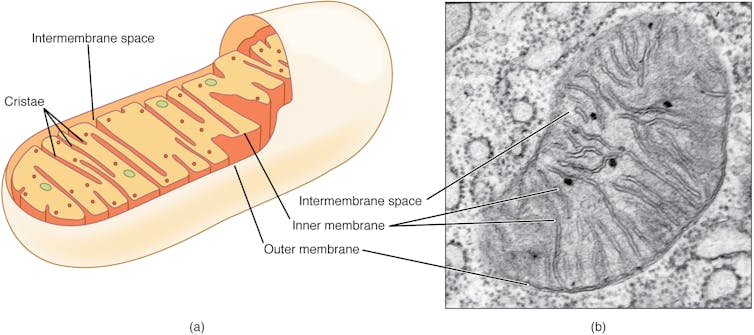
The immune system’s secret weapons
Neutrophils are the most abundant type of immune cell and serve as the immune system’s first responders. One of their key defense mechanisms is releasing neutrophil extracellular traps, or NETs – weblike structures composed of DNA and antimicrobial proteins. These sticky NETs trap and neutralize invading microbes, preventing their spread in the body.
Until recently, scientists believed that NET formation was primarily triggered by cellular stress and damage. However, our study found that mitochondria can detect a specific bacterial byproduct – lactate – and use that signal to initiate NET formation.
Lactate is commonly associated with muscle fatigue in people. But in the context of bacterial infections, it plays a different role. Many bacteria release lactate as part of their own energy production. My team found that once bacteria are engulfed by a compartment of the cell called the phagosome, neutrophils can sense the presence of this lactate.
Inside the phagosome, this lactate communicates to the neutrophil that bacteria are present and that the antibacterial processes are not sufficient to kill these pathogens. When the mitochondria in neutrophil cells detect this lactate, they start signaling for the cell to get rid of the NETs that have entrapped bacteria. Once the bacteria are released outside the cell, other immune cells can kill them.
When we blocked the mitochondria’s ability to sense lactate, neutrophils failed to produce NETs effectively. This meant bacteria were more likely to escape capture and proliferate, showing how crucial this mechanism is to immune defense. This process highlights an intricate dialogue between the bacteria’s metabolism and the host cell’s energy machinery.
What makes this finding surprising is that the mitochondria within cells are able to detect bacteria trapped in phagosomes, even though the microbes are enclosed in a separate space. Somehow, mitochondrial sensors can pick up cues from within these compartments – an impressive feat of cellular coordination.
Targeting mitochondria to fight infections
Our study is part of a growing field called immunometabolism, which explores how metabolism and immune function are deeply intertwined. Rather than viewing cellular metabolism as strictly a means to generate energy, researchers are now recognizing it as a central driver of immune decisions.
Mitochondria sit at the heart of this interaction. Their ability to sense, respond to and even shape the metabolic environment of a cell gives them a critical role in determining how and when immune responses are deployed.
For example, our findings provide a key reason why patients with a chronic autoimmune disease called systemic lupus erythematosus often suffer from recurrent infections. Mitochondria in the neutrophils of lupus patients fail to sense bacterial lactate properly. As a result, NET production was significantly reduced. This mitochondrial dysfunction could explain why lupus patients are more vulnerable to bacterial infections – even though their immune systems are constantly activated due to the disease.
This observation points to mitochondria’s central role in balancing immune responses. It connects two seemingly unrelated issues: immune overactivity, as seen in lupus, and immune weakness like increased susceptibility to infection. When mitochondria work correctly, they help neutrophils mount an effective, targeted attack on bacteria. But when mitochondria are impaired, this system breaks down.
Our discovery that mitochondria can sense bacterial lactate to trigger NET formation opens up new possibilities for treating infections. For instance, drugs that enhance mitochondrial sensing could boost NET production in people with weakened immune systems. On the flip side, for conditions where NETs contribute to tissue damage – such as in severe COVID-19 or autoimmune diseases – it might be beneficial to limit this response.
Additionally, our study raises the question of whether other immune cells use similar mechanisms to sense microbial metabolites, and whether other bacterial byproducts might serve as immune signals. Understanding these pathways in more detail could lead to new treatments that modulate immune responses more precisely, reducing collateral damage while preserving antimicrobial defenses.
Mitochondria are not just the powerhouses of the cell – they are the immune system’s watchtowers, alert to even the faintest metabolic signals of bacterial invaders. As researchers’ understanding of their roles expands, so too does our appreciation for the complexity – and adaptability – of our cellular defenses.
Andrew Monteith receives funding from the National Institute of Health.
Read These Next
Cuba’s speedboat shootout recalls long history of exile groups engaged in covert ops aimed at regime
From the 1960s onward, dissident Cubans in exile have sought to undermine the government in Havana −…
Drug company ads are easy to blame for misleading patients and raising costs, but research shows the
Officials and policymakers say direct-to-consumer drug advertising encourages patients to seek treatments…
Nanoparticles and artificial intelligence can help researchers detect pollutants in water, soil and
Tiny particles bounce light around in a unique way, a property that researchers are using to detect…