Science requires ethical oversight – without federal dollars, society’s health and safety are at ris
There are several steps between research on seemingly esoteric subjects and breakthrough medical treatments. Ethical oversight at every stage ensures science and society ultimately benefit.
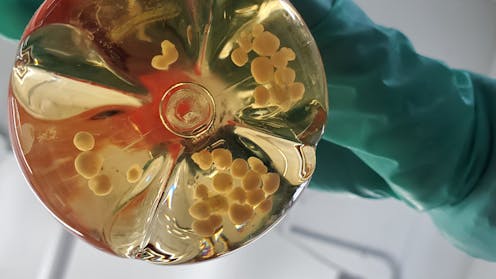
As the Trump administration continues to make significant cuts to NIH budgets and personnel and to freeze billions of dollars of funding to major research universities – citing ideological concerns – there’s more being threatened than just progress in science and medicine. Something valuable but often overlooked is also being hit hard: preventing research abuse.
The National Institutes of Health has been the world’s largest public funder of biomedical research. Its support helps translate basic science into biomedical therapies and technologies, providing funding for nearly all treatments approved by the Food and Drug Administration from 2010 to 2019. This enables the U.S. to lead global research while maintaining transparency and preventing research misconduct.
While the legality of directives to shrink the NIH is unclear, the Trump administration’s actions have already led to suspended clinical trials, institutional hiring freezes and layoffs, rescinded graduate student admissions, and canceled federal grant review meetings. Researchers at affected universities say that funding will delay or possibly eliminate ongoing studies on critical conditions like cancer and Alzheimer’s.
It is clear to us, as legal and bioethics scholars whose research often focuses on the ethical, legal and social implications of emerging biotechnologies, that these directives will have profoundly negative consequences for medical research and human health, with ripple effects that will last decades. Our scholarship demonstrates that in order to contribute to knowledge and, ultimately, to biomedical treatments, medical research at every stage depends on significant infrastructure support and ethical oversight.
Our recent focus on brain organoid research – 3D lab models grown from human stem cells that simulate brain structure and function – shows how federal support for research is key to not only promote innovation, but to protect participants and future patients.
History of NIH and research ethics
The National Institutes of Health began as a one-room laboratory within the Marine Hospital Service in 1887. After World War I, chemists involved in the war effort sought to apply their knowledge to medicine. They partnered with Louisiana Sen. Joseph E. Ransdell who, motivated by the devastation of malaria, yellow fever and the 1928 influenza pandemic, introduced federal legislation to support basic research and fund fellowships focusing on solving medical problems.
By World War II, biomedical advances like surgical techniques and antibiotics had proved vital on the battlefield. Survival rates increased from 4% during World War I to 50% in World War II. Congress passed the 1944 Public Health Services Act to expand NIH’s authority to fund biomedical research at public and private institutions. President Franklin D. Roosevelt called it “as sound an investment as any Government can make; the dividends are payable in human life and health.”
As science advanced, so did the need for guardrails. After World War II, among the top Nazi leaders prosecuted for war crimes were physicians who conducted experiments on people without consent, such as exposure to hypothermia and infectious disease. The verdicts of these Doctors’ Trials included 10 points about ethical human research that became the Nuremberg Code, emphasizing voluntary consent to participation, societal benefit as the goal of human research, and significant limitations on permissible risks of harm. The World Medical Association established complementary international guidelines for physician-researchers in the 1964 Declaration of Helsinki.

In the 1970s, information about the Tuskegee study – a deceptive and unethical 40-year study of untreated syphilis in Black men – came to light. The researchers told study participants they would be given treatment but did not give them medication. They also prevented participants from accessing a cure when it became available in order to study the disease as it progressed. The men enrolled in the study experienced significant health problems, including blindness, mental impairment and death.
The public outrage that followed starkly demonstrated that the U.S. couldn’t simply rely on international guidelines but needed federal standards on research ethics. As a result, the National Research Act of 1974 led to the Belmont Report, which identified ethical principles essential to human research: respect for persons, beneficence and justice.
Federal regulations reinforced these principles by requiring all federally funded research to comply with rigorous ethical standards for human research. By prohibiting financial conflicts of interest and by implementing an independent ethics review process, new policies helped ensure that federally supported research has scientific and social value, is scientifically valid, fairly selects and adequately protects participants.
These standards and recommendations guide both federally and nonfederally funded research today. The breadth of NIH’s mandate and budget has provided not only the essential structure for research oversight, but also key resources for ethics consultation and advice.
Brain organoids and the need for ethical inquiry
Biomedical research on cell and animal models requires extensive ethics oversight systems that complement those for human research. Our research on the ethical and policy issues of human brain organoid research provides a good example of the complexities of biomedical research and the infrastructure and oversight mechanisms necessary to support it.
Organoid research is increasing in importance, as the FDA wants to expand its use as an alternative to using animals to test new drugs before administering them to humans. Because these models can simulate brain structure and function, brain organoid research is integral to developing and testing potential treatments for brain diseases and conditions like Alzheimer’s, Parkinson’s and cancer. Brain organoids are also useful for personalized and regenerative medicine, artificial intelligence, brain-computer interfaces and other biotechnologies.
Brain organoids are built on knowledge about the fundamentals of biology that was developed primarily in universities receiving federal funding. Organoid technology began in 1907 with research on sponge cells, and continued in the 1980s with advances in stem cell research. Since researchers generated the first human organoid in 2009, the field has rapidly expanded.
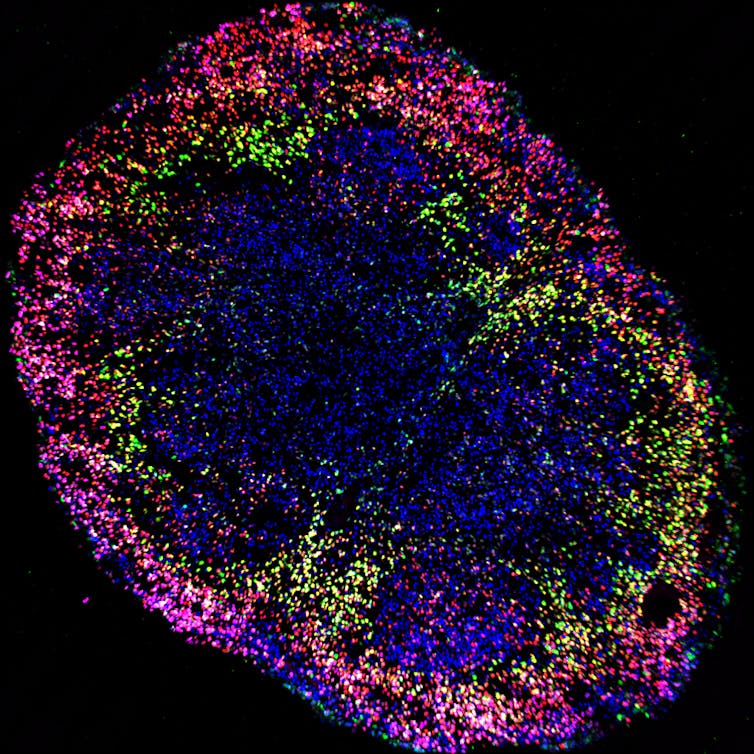
These advances were only possible through federally supported research infrastructure, which helps ensure the quality of all biomedical research. Indirect costs cover operational expenses necessary to maintain research safety and ethics, including utilities, administrative support, biohazard handling and regulatory compliance. In these ways, federally supported research infrastructure protects and promotes the scientific and ethical value of biotechnologies like brain organoids.
Brain organoid research requires significant scientific and ethical inquiry to safely reach its future potential. It raises potential moral and legal questions about donor consent, the extent to which organoids should be grown and how they should be disposed, and consciousness and personhood. As science progresses, infrastructure for oversight can help ensure these ethical and societal issues are addressed.
New frontiers in scientific research
Since World War II, there has been bipartisan support for scientific innovation, in part because it is an economic and national security imperative. As Harvard University President Alan Garber recently wrote, “[n]ew frontiers beckon us with the prospect of life-changing advances. … For the government to retreat from these partnerships now risks not only the health and well-being of millions of individuals but also the economic security and vitality of our nation.”
Cuts to research overhead may seem like easy savings, but it fails to account for the infrastructure that provides essential support for scientific innovation. The investment the NIH has put into academic research is significantly paid forward, adding nearly US$95 billion to local economies in fiscal year 2024, or $2.46 for every $1 of grant funding. NIH funding had also supported over 407,700 jobs that year.
President Donald Trump pledged to “unleash the power of American innovation” to battle brain-based diseases when he accepted his second Republican nomination for president. Around 6.7 million Americans live with Alzheimer’s, and over a million more suffer from Parkinson’s. Hundreds of thousands of Americans are diagnosed with aggressive brain cancers each year, and 20% of the population experiences varying forms of mental illness at any one time. These numbers are expected to grow considerably, possibly doubling by 2050.
Organoid research is just one of the essential components in the process of learning about the brain and using that knowledge to find better treatment for diseases affecting the brain.
Science benefits society only if it is rigorous, ethically conducted and fairly funded. Current NIH policy directives and steep cuts to the agency’s size and budget, along with attacks on universities, undermine globally shared goals of increasing understanding and improving human health.
The federal system of overseeing and funding biomedical science may need a scalpel, but to defund efforts based on “efficiency” is to wield a chainsaw.
The authors do not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
Read These Next
Kansas revoked transgender people’s IDs overnight – researchers anticipate cascading health and soci
With invalid driver’s licenses and birth certificates, transgender people are at risk for more than…
Cuba’s speedboat shootout recalls long history of exile groups engaged in covert ops aimed at regime
From the 1960s onward, dissident Cubans in exile have sought to undermine the government in Havana −…
Drug company ads are easy to blame for misleading patients and raising costs, but research shows the
Officials and policymakers say direct-to-consumer drug advertising encourages patients to seek treatments…




