Drugs that aren’t antibiotics can also kill bacteria − new method pinpoints how
There are many ways to kill microbes that cause dangerous infections. Combining genetic screening with machine learning can help researchers identify new antimicrobials.

Human history was forever changed with the discovery of antibiotics in 1928. Infectious diseases such as pneumonia, tuberculosis and sepsis were widespread and lethal until penicillin made them treatable. Surgical procedures that once came with a high risk of infection became safer and more routine. Antibiotics marked a triumphant moment in science that transformed medical practice and saved countless lives.
But antibiotics have an inherent caveat: When overused, bacteria can evolve resistance to these drugs. The World Health Organization estimated that these superbugs caused 1.27 million deaths around the world in 2019 and will likely become an increasing threat to global public health in the coming years.
New discoveries are helping scientists face this challenge in innovative ways. Studies have found that nearly a quarter of drugs that aren’t normally prescribed as antibiotics, such as medications used to treat cancer, diabetes and depression, can kill bacteria at doses typically prescribed for people.
Understanding the mechanisms underlying how certain drugs are toxic to bacteria may have far-reaching implications for medicine. If nonantibiotic drugs target bacteria in different ways from standard antibiotics, they could serve as leads in developing new antibiotics. But if nonantibiotics kill bacteria in similar ways to known antibiotics, their prolonged use, such as in the treatment of chronic disease, might inadvertently promote antibiotic resistance.
In our recently published research, my colleagues and I developed a new machine learning method that not only identified how nonantibiotics kill bacteria but can also help find new bacterial targets for antibiotics.
New ways of killing bacteria
Numerous scientists and physicians around the world are tackling the problem of drug resistance, including me and my colleagues in the Mitchell Lab at UMass Chan Medical School. We use the genetics of bacteria to study which mutations make bacteria more resistant or more sensitive to drugs.
When my team and I learned about the widespread antibacterial activity of nonantibiotics, we were consumed by the challenge it posed: figuring out how these drugs kill bacteria.
To answer this question, I used a genetic screening technique my colleagues recently developed to study how anticancer drugs target bacteria. This method identifies which specific genes and cellular processes change when bacteria mutate. Monitoring how these changes influence the survival of bacteria allows researchers to infer the mechanisms these drugs use to kill bacteria.
I collected and analyzed almost 2 million instances of toxicity between 200 drugs and thousands of mutant bacteria. Using a machine learning algorithm I developed to deduce similarities between different drugs, I grouped the drugs together in a network based on how they affected the mutant bacteria.
My maps clearly showed that known antibiotics were tightly grouped together by their known classes of killing mechanisms. For example, all antibiotics that target the cell wall – the thick protective layer surrounding bacterial cells – were grouped together and well separated from antibiotics that interfere with bacteria’s DNA replication.
Intriguingly, when I added nonantibiotic drugs to my analysis, they formed separate hubs from antibiotics. This indicates that nonantibiotic and antibiotic drugs have different ways of killing bacterial cells. While these groupings don’t reveal how each drug specifically kills antibiotics, they show that those clustered together likely work in similar ways.
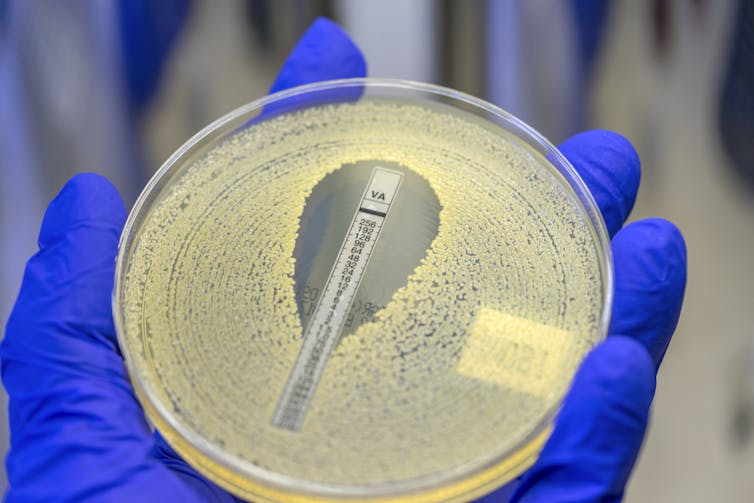
The last piece of the puzzle – whether we could find new drug targets in bacteria to kill them – came from the research of my colleague Carmen Li. She grew hundreds of generations of bacteria that were exposed to different nonantibiotic drugs normally prescribed to treat anxiety, parasite infections and cancer. Sequencing the genomes of bacteria that evolved and adapted to the presence of these drugs allowed us to pinpoint the specific bacterial protein that triclabendazole – a drug used to treat parasite infections – targets to kill the bacteria. Importantly, current antibiotics don’t typically target this protein.
Additionally, we found that two other nonantibiotics that used a similar mechanism as triclabendazole also target the same protein. This demonstrated the power of my drug similarity maps to identify drugs with similar killing mechanisms, even when that mechanism was yet unknown.
Helping antibiotic discovery
Our findings open multiple opportunities for researchers to study how nonantibiotic drugs work differently from standard antibiotics. Our method of mapping and testing drugs also has the potential to address a critical bottleneck in developing antibiotics.
Searching for new antibiotics typically involves sinking considerable resources into screening thousands of chemicals that kill bacteria and figuring out how they work. Most of these chemicals are found to work similarly to existing antibiotics and are discarded.
Our work shows that combining genetic screening with machine learning can help uncover the chemical needle in the haystack that can kill bacteria in ways researchers haven’t used before. There are different ways to kill bacteria we haven’t exploited yet, and there are still roads we can take to fight the threat of bacterial infections and antibiotic resistance.
Mariana Noto Guillen does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Read These Next
Enforcing Prohibition with a massive new federal force of poorly trained agents didn’t go so well in
Both Prohibition and current mass deportation efforts were hastily built, staffed by people permitted…
Menstrual pads and tampons can contain toxic substances – here’s what to know about this emerging he
Heavy metals, phthalates and other potentially harmful chemicals have been detected in a range of menstrual…
I’m a philosopher who tries to see the best in others – but I know there are limits
Seeing someone charitably requires a balancing act between taking them seriously and trying to see the…