Blood tests for Alzheimer’s: Two experts on why new studies are encouraging
Alzheimer's is one of the most dreaded and difficult diseases, and it has been notoriously difficult to diagnose. Could that change in the coming years with the advent of new blood tests?

Many people who have problems with their memory, especially if they are elderly, worry that they have Alzheimer’s disease, which afflicts at least 5.5 million people in the U.S. and brings tremendous burdens to families as well. This concern is paramount among those who have seen a family member, friend or colleague develop this insidious progressive disease.
Now, there is a real possibility that blood tests may aid in firming up a clinical diagnosis of Alzheimer’s disease, and that additional blood tests may help to determine how the disease will progress. Studies presented at the Alzheimer’s Association International Conference in Los Angeles in July demonstrated the utility of various blood tests for Alzheimer’s disease.
The studies also suggested that these tests could identify individuals with the underlying AD pathology years before patients show symptoms. This could allow people who have positive tests to enroll in “prevention trials” that could delay or even prevent the disease.
Both of us are physician-scientists who have studied Alzheimer’s and other neurodegenerative diseases for many decades. The field has gained a great deal of knowledge about AD, but it has been challenging to develop novel therapies.
We remain cautiously optimistic that with increased federal funding and concerted efforts of thousands of physicians, scientists, patients and patient advocates, that breakthroughs in terms in treatment are more likely to occur than ever before. Although not therapies, we believe these blood tests can help make tests of new therapeutics more powerful.
A tough disease even to diagnose
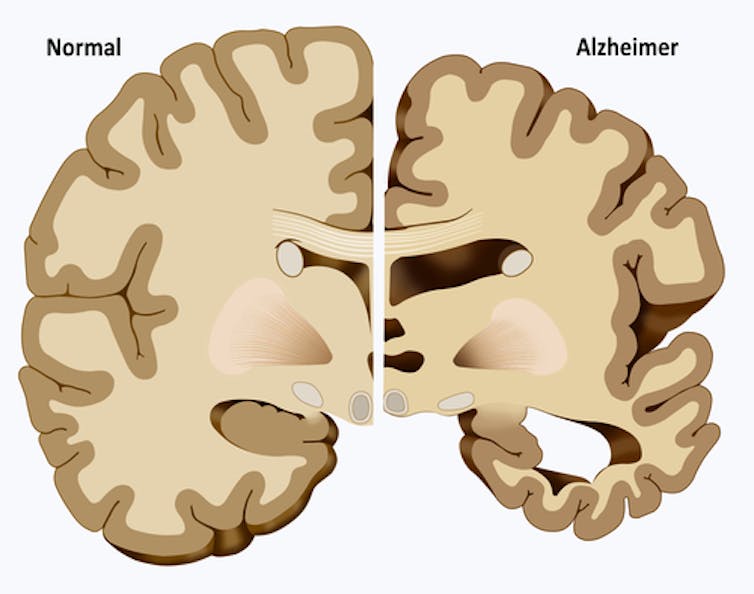
It has never been easy to know whether a person has Alzheimer’s. Just 30 years ago, even the best neurologists would get the diagnosis wrong about one in four times. Diagnosis was even harder in people over 80, where the changes in thinking and memory with aging were not always easy to separate from AD symptoms.
Until this century, the only definitive diagnosis of AD occurred after death, in a brain autopsy. Clinicians could say for sure a person had Alzheimer’s if they found certain levels of two lesions, or areas of abnormal tissue, at autopsy. Those two lesions are beta-amyloid plaques and neurofibrillary tangles. But no blood or other body fluid tests or imaging studies existed that could be done on a living person and show they had AD.
And it was not unusual to find, following autopsy, that someone clinically diagnosed with Alzheimer’s disease had another type of neurodegenerative disease, or disease related to blood vessels in the brain, or some combination of these.
Over the last two decades, however, the medical field has made progress in detecting the disease by identifying diagnostic biomarkers, or biological signs of disease. MRI scans helped by showing shrinkage of the areas of the brain that underlie memory. But they are not specific for AD.
One of the key biomarkers is the amyloid protein, found in plaques. Another is the tau protein, found in tangles.
Once these biomarkers were identified, doctors could test patients to see if either or both were elevated in patients in whom they suspected Alzheimer’s. But the testing has not been easy or cheap.
One way was a spinal tap, whereby doctors could obtain cerebrospinal fluid, the fluid around your brain and spine, and measure levels of tau and amyloid, which change if the disease is present. While doctors consider this procedure safe and routine, it is not a favorite among patients.
Another method involves imaging the brain using a positron emission tomography (PET) scan following administration of compounds (amyloid or tau “tracers”) that bind one of the proteins that accumulates in the Alzheimer’s diseased brain. The amyloid scans came first, about 15 years ago, and revolutionized research in AD; tau scans are still developing, but they work to reveal neurofibrillary tangles.
Scans, taps and limitations
While spinal taps and PET scans are useful, they both have limitations. People do not exactly look forward to getting a spinal tap. The PET imaging studies involve administration of a slightly radioactive compound, which is not significant. But although extremely safe, individual PET scans are expensive – typically from US$3,000 up – and Medicare does not pay for them.
Tau PET tracers are in early development, but will be useful for research and to increase knowledge gained from clinical trials testing new drugs.
The impact of these advances is huge, especially in research or clinical trials, where maximum likelihood of the right diagnosis is necessary. But the medical community still needs a more convenient, less expensive, less “invasive” way to diagnose Alzheimer’s. This really means … a blood test.
For years, efforts to find such an easily obtainable AD diagnostic biomarker in the blood came up empty. A number of reported “breakthroughs” claiming discovery of a novel diagnostic test, but none ever panned out.
Possible blood tests emerge

Now, several publications and numerous presentations at the recent conference demonstrated hopeful news. Blood tests to measure amyloid protein, and possibly tau protein, are becoming much more sensitive and reliable enough to become routine aids in helping to diagnose AD.
These various tests are at different stages of validation – assuring they’re accurate across many different populations. And, for each protein, there are several different methods for making the blood measurements. So there is still work to do before any of these tests will be widely used in medical practice. Predictions are difficult, but without any more difficulties, we hope they can be applied in a few years.
To be useful, these tests have to be nearly perfect predictors. They aren’t there yet; so far, they seem to get it right about 85-90+% of the time. This accuracy will be even more important if they’re to be used to identify people for new therapies.
It’s still too early to tell if one AD blood test will prove better than the others. However, given the long road to this point, the research community is excited about the possibilities.
The tests measuring amyloid actually measure the ratio of two different sizes of the amyloid peptide – similar to using the ratio of HDL to LDL blood cholesterol to evaluate lipids. If the ratio of the amyloid is decreasing in blood, it is accumulating in the brain, even before AD symptoms emerge. Their first use, however, will be in diagnosis of people with symptoms.
What the blood assays for the tau protein, the main component of tangles in the brain, tell us is little less certain. Most in the field believe, however, that they will may provide information on the stage or progression of the disease.
Collectively, these tests mark real progress. More certain, earlier and cost-effective diagnostic aids will help all of us reach our goal of finding novel treatments that can better treat the clinical symptoms of AD and/or delay its development.
Dr. Todd Golde is a co-founder of Lacerta Therapeutics, Inc. and serves on their scientific advisory board (SAB). He is on the SAB for Promis Neuroscience, Inc. In the past he has served, ad hoc, on SABs related to neurodegenerative disease programs for Eli Lilly, Novartis, Bristol Myers Squib, Abbvie, Lundbeck, Biogen and Pfizer. He is co-editor in chief of Alzheimer’s Research and Therapy for which he receives an honorarium. He has served on the medical and scientific advisory board for the Alzheimer’s Association. He serves as a scientific advisor and participates in grant reviews for BrightFocus Foundation and the American Federation for Aging Research. He is a co-inventor on multiple patents and patent applications relating to AD therapeutics. He currently receives funding from the NIH.
Steven DeKosky receives grant funding from the National Institute of Aging, serves as a consultant for Amgen, Biogen, and Cognition Therapeutics, and serves as editor for dementia for Up-To-Date, a point-of-care electronic textbook. He has chaired study sections for the NIH, served on two NIH councils, for the National Center for Complementary and Alternative Medicine (now the National Center for Complementary and Integrative Health; NCCIH) and the Director's Council (Council of Councils). He has served on the board of the Alzheimer's Association and chaired their Medical and Scientific Advisory Council, as well as chairing the Medical and Scientific Advisory Panel of Alzheimer's Disease International.
Read These Next
How does Iran go about selecting a new supreme leader? And who is in the running?
Media reports have cast the son of slain Ayatollah Ali Khamenei as a top candidate for supreme leader.…
We designed an AI tutor that helps college students reason rather than give them answers
A 2025 study shows that an AI-based tutor improves learning when it prompts reasoning and is paired…
GLP-1 drugs may fight addiction across every major substance, according to a study of 600,000 people
GLP-1 drugs are the first medication to show promise for treating addiction to a wide range of substances.