Mapping the 100 trillion cells that make up your body
First, scientists wanted to decode all three billions units of the human genome. Now, a new effort will identify all the cell types in the human body to discover the roots of diseases, like diabetes.
There are about 100 trillion cells that make up the human body. A new megascience endeavor will catalog and image each of the 200 or more types of cells from the 80 known organs and identify the genes that are active in these cells.
This new effort follows on the heels of the Human Genome Project that engulfed biology during the 1990s and early 2000s. Now scientists have conceived a new and exciting challenge: to create a cellular map of the entire human body, a project called the Human BioMolecular Atlas Program, or HuBMAP. The University of Florida is one of five participating tissue mapping centers. Here at the UF Center we are charged with mapping the thymus, lymph node, and spleen – all key components of the immune system.
I have been studying Type 1 diabetes, or juvenile diabetes, for nearly 35 years and along with my other colleagues at the UF Diabetes Institute have been trying to find a way to prevent and cure the disease. This has been a challenge as until recently, because we didn’t know what caused Type 1 diabetes.
Our goal as a tissue mapping center is to identify the unique types of cells, which proteins they produce and which genes are turned on, and build a virtual three-dimensional model of each organ. This map will inform the research of many diseases, including Type 1 diabetes.
Why is understanding the causes of Type 1 diabetes important?
We know that Type 1 diabetes is a so-called “autoimmune disorder.” In Type 1 diabetes, immune cells known as “T lymphocytes” are thought to destroy the pancreatic beta cells that are responsible for producing insulin, which regulates the level of sugar in our blood.
Just over a decade ago, frustrated by the inability to prevent and cure the disease, I started an initiative to collect human pancreases from organ donors with Type 1 diabetes as well as those without the disease. The latter group was collected to provide an understanding of a “normal” healthy pancreas. To date, we have collected the pancreas from more than 500 individuals. We have distributed these tissues to some 230 projects in 21 countries around the world. The results of this effort have led to new discoveries that have rewritten our understanding about how this disease develops.
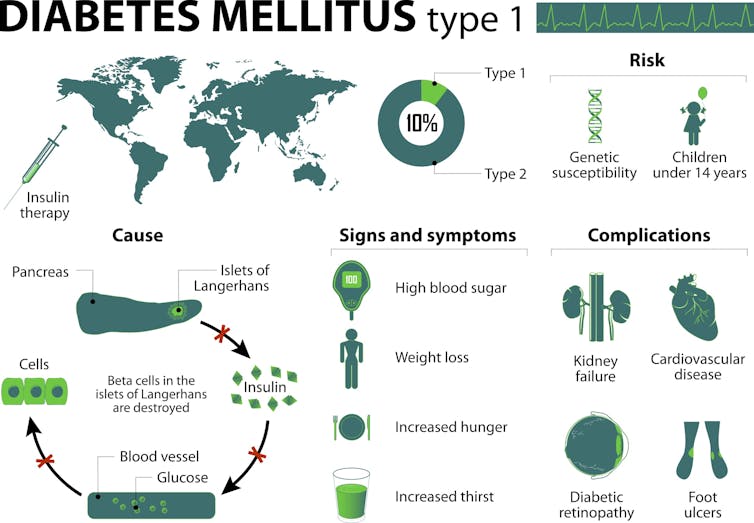
Patients diagnosed with Type 1 diabetes, some 25,000 per year in the U.S. alone, face a lifelong dependence on daily insulin injections in order to survive and have a high risk of developing long-term medical complications including blindness, kidney disease, numb feet, limb amputations and cardiovascular disease. Today, it is estimated that nearly 1.25 million people in the U.S. live with this disorder.
As upsetting as these complications are for individuals with the disease, perhaps even more daunting are the many daily lifestyle factors that must be controlled or accounted for to keep the disease in check: monitoring carbohydrates, estimating exercise, evaluating blood sugar levels, and administering insulin to avoid both high and low blood glucose levels. These represent just a few of the daily disease associated challenges.
For these reasons, the goal of our collective research efforts at the UF Diabetes Institute has always been to understand what causes this disease. Knowing that would enable us to predict who is at risk, identify ways to prevent the progression of the disease, and develop a curative therapy.
Why study these organs?
Type 1 diabetes is but one of more than 80 known autoimmune diseases that, for reasons unknown, the immune system turns against itself. Beyond autoimmunity, immune responses are also a key constituent to health in terms of fighting cancer and infectious disease. From our experience studying the pancreas and Type 1 diabetes, we see great strides in understanding the role for immunity in each of these settings through mapping. It will allow for a deep dive of how the immune system works.
In a healthy individual, T cells only become active when responding to infection or cancer cells. But in those predisposed to autoimmune disease, certain T cells can become erroneously activated by “self” proteins, leading them to destroy healthy tissue.
In other circumstances – like cancer or infectious disease – the immune system fails to provide a robust enough response to be effective. Or cells of the immune system proliferate uncontrollably, leading to blood and lymphatic cancers like lymphomas and leukemias. This is why the thymus, spleen and lymph node are tissues of interest for those studying the healthy human immune system. Researchers need to understand the healthy baseline for all these organs so that we can recognize when things begin to malfunction and change, leading to autoimmune disease, cancer and infectious disease. Expressed another way, we first need to understand what constitutes the normal lymphatic system throughout the human lifespan.
Why is defining normal important?
You might wonder where exactly we get these normal cells. As we have done over the past 11 years, we will obtain transplant-grade human tissues from deceased organ donors through Organ Procurement Organizations, after a family member or legal executor provides informed consent. Given at a time of grieving, these precious anatomical gifts, which in the case of spleen, thymus and lymph node, are not usable for lifesaving transplantation procedures, provide an inimitable resource for scientific investigation and discovery.
Only tissues considered “normal” – unaffected by known or observable pathologies – will be included in these initial studies. We will be collecting tissues from donors ranging from infants to adults up to 70 years old. We hope this will provide insights into how age alters the types and health of all the cells in each organ.
At the UF Diabetes Institute a multidisciplinary team including cellular and molecular biologists, hematopathologists who study clinical lymphatic samples, biomedical engineers, immunologists and many others will collaborate for the HuBMAP program. Indeed, the UF tissue mapping center will collaborate extensively with a global network of experts in cutting-edge microscopy and data collection.
We are establishing an imaging pipeline to detect dozens of protein and RNA molecules that characterize nerve, blood vessel, the supportive tissue known as stroma, and immune cells from slices of tissue, using eight different forms of microscopy.
Within HuBMAP’s first two years, we plan to map the spleen, thymus and lymph node from 11 organ donors.
We expect that the resulting data will reveal new cell types, molecular and cellular structures, cell-cell interactions and their functional implications in human anatomy and physiology. Hence, the high-resolution, three-dimensional Human BioMolecular Atlas Program is expected to facilitate discovery.
As I hit my late 50s in life, the number of colleagues, friends and family members that are impacted by disease increases annually. I also recently became a grandfather. I would like to think what we propose to do will have a dramatic impact on human health for both current and future generations. That would be a legacy gift.
Mark Atkinson receives funding from the National Institutes of Health, JDRF, American Diabetes Association, and The Leona M. and Harry B. Helmsley Charitable Trust. He is the President of Insulin for Life-USA, a not-for-profit organization seeking to provide type 1 diabetes management supplies to those in need.
Read These Next
The inspiring and tragic story of Mabel Stark, America’s most famous female tiger trainer
Long before Joe Exotic became Tiger King, Mabel Stark reigned as Tiger Queen.
Formerly incarcerated Black men say they’re ‘doing OK’ while trying to cope with depression and PTSD
A nurse scientist interviewed 29 formerly incarcerated Black men in Philadelphia to understand…
Ayatollah Ali Khamenei’s killing plays into Shiite Islam’s reverence for martyrs, but not for all Ir
Khamenei was a deeply polarizing figure in Iran – perceived by some as a martyr and others as an oppressor.






