Approval of first ‘RNA interference’ drug – why the excitement?
The FDA just a approved a new type of drug called siRNA. Here's how it works and why everyone is so excited about it.
Small interfering RNA sounds like something from a science fiction novel rather than a revolutionary type of medicine. But this odd-sounding new drug offers a novel strategy for treating disease by targeting the root cause rather than just the symptoms. This is an exciting approach because it enhances the effectiveness of the treatment and reduces side effects.
The Food and Drug Administration recently approved the very first therapeutic small interfering RNA (siRNA), Onpattro (patisiran), to treat nerve damage caused by a rare disease called hereditary transthyretin-mediated amyloidosis (hATTR). Hereditary transthyretin-mediated amyloidosis affects about 50,000 people worldwide. The major cause is the buildup of a protein called amyloid in the peripheral nerves, heart and other organs. Small interfering RNA was first described in 1998 and its discoverers were awarded the Nobel prize in physiology or medicine in 2006. Twenty years later the discovery has been translated into a new form of medicine.
Proteins make up the largest structural and functional portion of our cells and tissues. The instructions to make a particular protein is encoded in our DNA. In order for the protein to be made, DNA must first be transcribed into an intermediate molecule called messenger RNA, which is then translated into a protein. Simply put, DNA makes RNA makes protein.
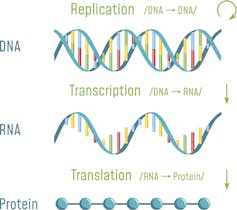
A disease such as hATTR is caused by excessive amyloid protein. One solution to overcoming these defects is to limit the protein from being made in the first place.
That’s where siRNA comes in.
The beauty of RNA drugs like Onpattro lies in its specificity. Onpattro is a small stretch of RNA that “interferes” with its counterpart mRNA. If the Earth represents a cell and all of the train tracks on Earth represents the RNA within that cell, Onpattro works by inhibiting a specific sequence of RNA; disrupting a stretch of train track that connects Pittsburgh and Cleveland. After Onpattro binds to its target, molecular scissors within the cell precisely cut the RNA preventing production of the amyloid protein.
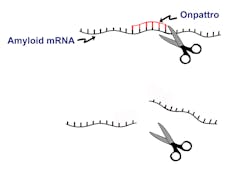
Although this siRNA strategy seems elegant and precise, it is not without challenges. Delivering the drug to the proper location within the body and the high costs associated with producing these complex therapeutics are hurdles that must be overcome before these drugs are widely adopted.
To improve the delivery of the drug to its target in patients, it must be protected from destruction in the blood stream. To accomplish this, Onpattro has been encapsulated in a protective fatty nanoparticle. The cost of treating hATTR with Onpattro is estimated at US$450,000 per patient per year.
There is also continued research and development of RNA-based therapies to treat disorders caused by changes in the cell triggered by a close cousin of siRNA, called microRNA. RNA drugs offer a new weapon in the arsenal to treat disease by blocking the production of specific cellular proteins. With the approval of Onpattro, one can anticipate applying RNA therapeutics to treat other genetic diseases such as cancer, which is partly caused by excessive or hyperactive proteins.
Thomas Schmittgen receives funding from the National Institutes of Health.
Read These Next
1 protein to rule them all – why crowning the protein that makes jellyfish glow green as a model can
Researchers have been studying tens of thousands of proteins and even more variations without a yardstick…
Florida’s proposed cuts to AIDS drug program threaten patient care and public health
Scaling back Florida’s AIDS Drug Assistance Program could mean a resurgence of HIV/AIDS and increased…
Violent aftermath of Mexico’s ‘El Mencho’ killing follows pattern of other high-profile cartel hits
Members of the Jalisco New Generation Cartel have set up roadblocks and attacked property and security…