Philip Morris hides data in plain sight on dangers of new heat-not-burn product
Philip Morris has applied to the FDA to market a product that it says is safer than a cigarette, but its own data show that is not true.

For as long as smoking has been known to cause cancer and other diseases, Big Tobacco has worked to avoid the truth about its deadly and highly addictive products.
Nicotine is the addictive drug in tobacco. Burning the tobacco generates an aerosol of ultrafine particles that carries nicotine deep into smokers’ lungs, where it is absorbed and rapidly reaches the brain. That burning yields toxic chemicals that cause disease.
Ever since people started understanding in the 1950s that smoking kills, millions have struggled to stop smoking. The tobacco companies, desperate to keep and expand their customers, have been trying to make “safer cigarettes” since the 1960s.
They have also developed products that avoided burning, including products that heat the tobacco without combustion, e-cigarettes and even nicotine replacement therapy.
Philip Morris International’s IQOS is the latest entry into this sweepstakes.
IQOS is a hand-held electric device that generates its nicotine aerosol by heating a stick of ground tobacco and chemicals without setting the tobacco on fire. IQOS does not burn the tobacco, so it produces fewer toxic chemicals than a cigarette.
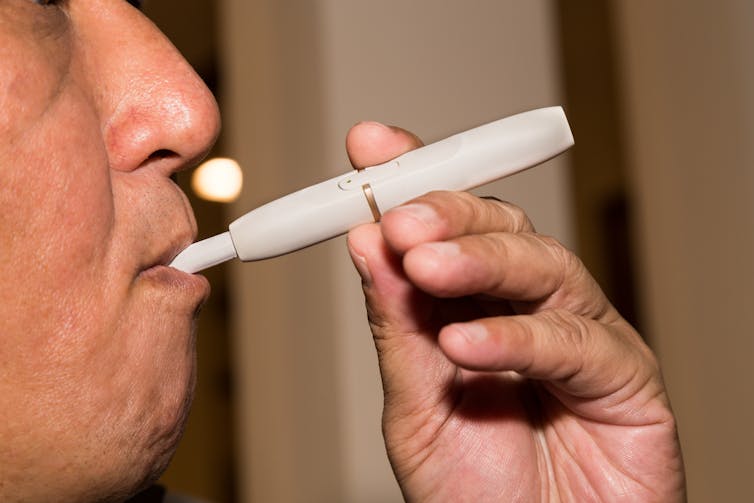
Because IQOS is a new tobacco product, it needs the Food and Drug Administration’s approval to sell it in the United States. Philip Morris submitted its massive application to the FDA on May 24, 2017. As required by law, FDA has made most of the application available for the public to review. The FDA will then consider the comments to determine if IQOS “as it is actually used by consumers, will significantly reduce harm and the risk of tobacco-related disease to individual users” and to the population as a whole. FDA can approve IQOS only if it meets this standard.
As someone who has worked in tobacco control for decades, I plowed through the application to see what information Philip Morris presented. To my surprise, I found (and told the FDA) that Philip Morris’s own application shows that in American people there is no statistical difference in the harm caused by IQOS product and traditional cigarettes.
Bad stuff gets in your lungs either way
Like cigarettes (and e-cigarettes), IQOS uses an aerosol of ultrafine particles to deliver the nicotine. These ultrafine particles cause heart and lung disease.
And the adverse health effects of these particles and many of the other toxins do not drop in proportion to reducing the dose, so even low levels of exposure can be dangerous. This effect is why smoke-free environments are followed by big drops in heart attacks despite the fact that secondhand smokers breathe in much less smoke that the smokers.
Nevertheless, Philip Morris is aggressively marketing IQOS all over the world on the grounds that it is not as bad as a cigarette because “the tobacco is heated and not burned, the levels of harmful chemicals are significantly reduced compared to cigarette smoke.”
Independent research has found higher levels than Philip Morris claims. Fewer toxic chemicals, however, do not necessarily translate into lower harm.
In the United States, Philip Morris wants to sell IQOS with claims that “Scientific studies have shown that switching completely from cigarettes to the IQOS system can reduce the risks of tobacco-related diseases” and “Switching completely to IQOS presents less risk of harm than continuing to smoke cigarettes.”
To support these claims, Philip Morris’s application presents data on toxic chemicals and effects in animals. Most important, Philip Morris reports medical tests that doctors use to assess people’s health in people using IQOS.
These 24 medcial tests include blood (cholesterol, inflammation, oxidative stress), blood pressure and lung function. They are the most important information in the application because they represent direct evidence of how IQOS affects people who use them.
A health hazard by any other name
I closely examined Philip Morris’s results. They show that there is no statistically detectable difference between IQOS and conventional cigarettes in these medical tests in the Americans Philip Morris studied.
Like all medical tests, there is uncertainty in the results. This range of uncertainty is what statisticians call the 95 percent confidence interval and journalists call “the margin of error.”
For 23 of the medical tests, the margin of error in the tests to discern the difference between IQOS and conventional cigarettes included a zero (i.e., no difference). So neither we nor the FDA can be 95 percent confident that IQOS are better for people than conventional cigarettes in those cases.
Moreover, when using the conventional 95 percent confidence standard, one would expect 5 percent of the tests to yield false positives or 1 out of 24 tests. That is exactly what Philip Morris reported.
In other words, Philip Morris’s own data demonstrate that IQOS is no different from conventional cigarettes in terms of effects on these medical tests in American people.
Too hot to cook your turkey
This is not surprising, because IQOS heats the tobacco to 660° Fahrenheit (350° Celsius). That’s well below the 1,100°F for combustion, but it is still hot enough to cause chemical reactions known as pyrolysis. Pyrolysis is what turns a turkey baked at 350°F into Thanksgiving dinner. Imagine if you had eaten a turkey cooked at IQOS’s 660°F!
These conclusions are based on taking Philip Morris’s results at face value, ignoring the fact that the tobacco industry, including Philip Morris, has a long history of manipulating scientific study designs and statistical analysis to get the results they want.
And there is already independent evidence that IQOS compromises functioning of arteries, a key risk factor for heart disease and heart attacks, as badly as a cigarette.
Because Philip Morris’s medical tests in humans failed to show that IQOS “as it is actually used by consumers, will significantly reduce harm and the risk of tobacco-related disease to individual users,” I believe the FDA must deny Philip Morris’s application to protect the public health.
Philip Morris’s application did include one accurate statement: “The best way to reduce your risk of tobacco-related diseases is to completely quit tobacco use.” Of course, if people did that, Philip Morris would not make any more money from them.
Stanton Glantz receives funding from the National Cancer Institute, the National Institute of Drug Abuse, and the Truth Initiative. He is the principal investigator of the University of California Tobacco Center on Regulatory Science, which is funded by the National Cancer Institute in collaboration with the Food and Drug Administration. None of these agencies supported the writing of this article.
Read These Next
GLP-1 drugs may fight addiction across every major substance, according to a study of 600,000 people
GLP-1 drugs are the first medication to show promise for treating addiction to a wide range of substances.
Housing First helps people find permanent homes in Detroit − but HUD plans to divert funds to short-
Detroit’s homelessness response system could lose millions of dollars in federal funding for permanent…
Trauma patients recover faster when medical teams know each other well, new study finds
A new study from a Pittsburgh hospital finds that trauma patients recover faster when emergency medical…