The FDA's rule change requiring providers to inform women about breast density could lead to a flurr
Dense breast tissue is common and normal, but it can make cancer more difficult to detect. FDA requirements going into effect in September 2024 will dictate that patients be better informed about it.
The U.S. Food and Drug Administration finalized a regulation in early March 2023 that updates mammography reporting requirements. The new regulation goes into effect on Sept. 10, 2024, and will require that all women receive information about breast density following a mammogram. It will also require they be told in their mammogram report that dense breast tissue can mask cancer and make cancer more difficult to detect.
The Conversation asked Dr. Wendie A. Berg, professor of radiology at the University of Pittsburgh School of Medicine, to explain how the rule change could affect screening recommendations as well as the way people interpret their results.
What is breast density and why does it matter?
All breasts are made up of a mix of fat, milk glands and ducts. The glands are supported by fibrous tissue and ligaments, collectively called “fibroglandular tissue.” The more fibroglandular tissue a woman has, the “denser” her breast tissue.
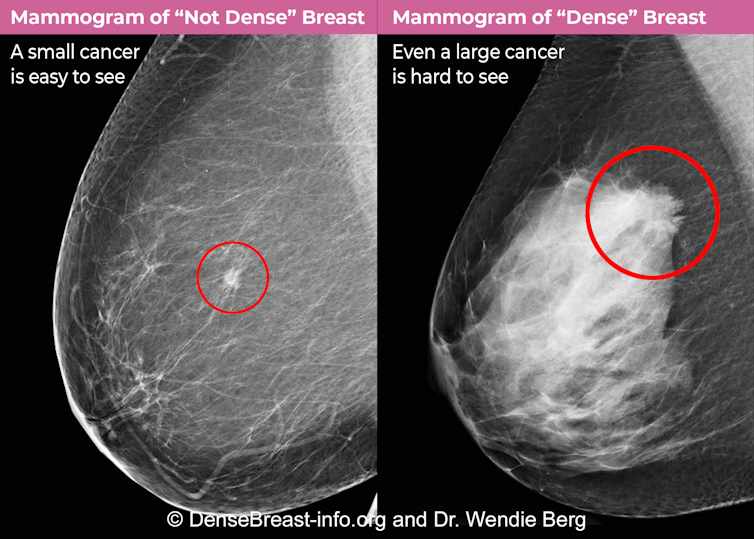
When a woman has a mammogram, the radiologist reviewing it will describe her breast density using one of four categories: A) fatty, B) scattered tissue, C) heterogeneously dense or D) extremely dense. Categories C and D are considered “dense” whereas categories A and B are “not dense.”
Dense breasts are normal and common. Over 50% of women have dense breasts before menopause, as do about 40% of women in their 50s and 30% of women in their 60s. Breasts can become less dense after menopause, but a woman with extremely dense breasts will likely continue to have dense breasts all her life.
Breast density matters for two reasons. Most importantly, dense breast tissue can hide cancer on a mammogram. About 40% of breast cancers will go unseen on mammography in the densest breasts, labeled “extremely dense breasts,” and about 25% will go undetected in heterogeneously dense breasts.
Secondly, dense tissue also increases the risk of developing breast cancer, with about fourfold the risk of breast cancer in extremely dense breasts compared with fatty breasts, and about twofold the risk compared to breasts with scattered tissue.
What does the FDA ruling entail?
Until now, 38 states plus Washington, D.C., have had varying laws about what to tell women about breast density. This has resulted in inconsistent levels of information being provided to U.S. women depending on where they live.
Beginning in September 2024, the FDA’s final rule creates a uniform national standard requiring that all women be informed in the mammogram results letter that their breasts are either “dense” or “not dense.” They will be told that dense tissue can hide cancer on a mammogram and that it also raises the risk of developing breast cancer.
The new regulations require that the specific density category be included in all mammography reports that go to the referring health care provider. Some states require the specific density category also be included in the patient results letter, and this information can be included, but must be separate from the language required by the FDA. The FDA notification cannot be altered in any way.
The FDA requirement also includes this sentence in the letter to women with “dense” breasts: “In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers.” Such “supplemental screening” deserves discussion.
How might this affect how patients respond to mammogram results?
Without some guidance on what to do about it, there is the potential for this information to cause some confusion and worry.
3D mammograms, also known as tomosynthesis, are becoming standard and are slightly better at detecting cancer, with fewer callbacks for extra testing for findings that turn out not to be cancer. Women with dense breasts should make sure to have routine screening with a 3D mammogram.
Deciding whether to pursue supplemental screening beyond an annual mammogram starting at age 40 depends on several considerations. These include breast density and other risk factors, potential benefits, downsides – such as additional testing for findings that turn out not to be cancer – insurance coverage and costs.
By age 30, all women should discuss their risk factors with their health care provider and consider genetic testing, if appropriate. This is because women considered to be “high risk” should start screening earlier and have MRI screening in addition to mammography, regardless of breast density.

Here is a list of some of the factors that would make a woman “high risk,” and good candidates for yearly screening with MRI up to ages 70 to 75, depending on overall health.
Women with disease-causing genetic variants, such as BRCA1 or BRCA2, or who have a mother, sister or daughter with a disease-causing variant, should start having yearly screening with MRI by age 25-30, and add mammography screening once they turn 30.
Women who received radiation therapy to the chest for prior cancer – usually Hodgkin lymphoma – before age 30 should start MRI screening eight years after treatment – but not before age 25 – and add mammography by the time they’re 30.
Women with an estimated lifetime risk of breast cancer of at least 20% should have annual MRIs, in addition to mammography. The most accurate estimates are from the Tyrer-Cuzick or IBIS model and include weight, height, breast density, family history, biopsy history and other risk factors. AI-based processing of mammograms alone may be even more accurate than risk models at predicting who will develop breast cancer in the next one to five years.
Annual MRI screenings are also recommended for women diagnosed with breast cancer prior to age 50 or women with dense breasts.
The European Society of Breast Imaging recommends that women with extremely dense breasts add MRI screening every 2 to 4 years from age 50 to 70 (with mammograms every 2 years).
Women with dense breasts, especially if they also have other risk factors such as family history of breast cancer or prior atypical biopsy, should consider adding screening MRI to their annual mammogram. But MRI requires lying in the tunnel of the magnet, which can be difficult for women with claustrophobia. It also requires intravenous contrast injection. Cancers become more visible with contrast because they have more and leakier blood vessels than normal tissue.
For women who cannot tolerate or access MRI, adding ultrasound to mammography can be considered, but MRI finds more cancers than ultrasound. Contrast-enhanced mammography is being evaluated as an alternative to MRI.
Will insurance cover additional screening tests?
Currently 15 states plus D.C. have laws requiring insurance coverage for supplemental breast cancer screening, but only New York, Connecticut and Illinois require such coverage without copays.
A federal insurance bill, the Find It Early Act, is being reintroduced by two U.S. representatives. This measure would ensure all health insurance plans cover screening and diagnostic breast imaging with no out-of-pocket costs.
This would include supplemental screening for women with dense breasts or at a higher risk for breast cancer, in accordance with National Comprehensive Cancer Network guidelines and the American College of Radiology’s Appropriateness Criteria.
Wendie A. Berg receives funding from the Breast Cancer Research Foundation and Pennsylvania Breast Cancer Coalition. She is voluntary Chief Scientific Advisor to www.DenseBreast-info.org.
Read These Next
War in Middle East brings uncertainty and higher energy costs to already weakening US economy
Risks for the US economy grow as the war in the Middle East continues to escalate.
China’s muted response over war in Iran reflects Beijing’s delicate calculus as a concerned onlooker
Beijing has denounced US-Israeli action in Iran, but has not rushed to come to the aid of its regional…
How Instagram addictiveness lawsuit could reshape social media – platform design meets product liabi
A lawsuit against Meta and Google avoids the issue of liability for content and focuses on allegations…





