Medication abortion could get harder to obtain – or easier: There's a new wave of post-Dobbs lawsuit
A rash of pending lawsuits raises questions about the FDA’s approval of mifepristone two decades ago, whether the drug can be legally mailed and the constitutional right to interstate commerce.
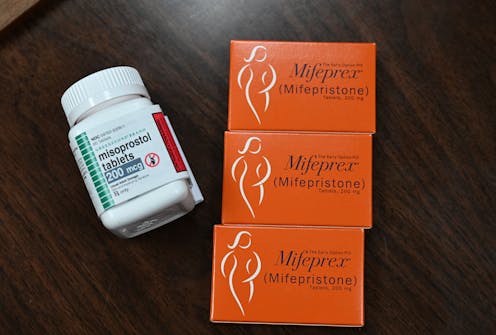
Medication abortion now accounts for more than half of all abortions in the United States.
Typically, patients take a two different pills: first mifepristone, then misoprostol.
Even though this option has been legally available for more than two decades, two recent events have raised legal questions about it. First, the Supreme Court’s Dobbs v. Jackson Women’s Health ruling overturned the constitutional right to abortion recognized in 1973 in Roe v. Wade. Second, in January 2023, the Food and Drug Administration decided that certified U.S. pharmacies could sell mifepristone by prescription.
The result is a raft of new legal battles over access to medication abortion.
Some congressional lawmakers seek to protect the right to access the pills through pharmacies and telehealth in states where abortion remains legal. At least three lawsuits are pending, and some states that have banned abortion altogether or have restricted access to it are vowing to block the new federal rules. South Dakota Governor Kristi Noem, for example, has threatened to prosecute any pharmacist who sells the pills in her state.
As experts on reproductive health and justice, we’re trying to untangle when and where mifepristone might be available and what these contradictory trends signal.
Prescribing abortion drugs
Who has the authority to determine when, how or whether abortion medication can be prescribed and sold?
Under its long-held, congressionally granted authority to regulate pharmaceutical products, the FDA approved mifepristone in 2000 after an extensive review demonstrated that the drug was safe and effective for early pregnancy termination.
From the beginning, the sale of mifepristone was tied to several safety requirements known as a risk evaluation and mitigation strategy. Initially, the drug had to be dispensed by certified medical providers in person.
But in late 2021, the FDA concluded that was no longer necessary for patient safety. Today, the pill, in its original or generic form, is approved for use up to 10 weeks’ gestation and can be dispensed by a certified prescriber or pharmacy.
Recent lawsuits challenge the scope of the FDA’s authority to regulate the sale of mifepristone.
In one, GenBioPro, a drug company that makes generic mifepristone, sued officials in West Virginia, claiming that the state’s abortion ban impedes its sales. GenBioPro argues that the ban contradicts FDA’s approval of and safety requirements for mifepristone, setting up a conflict between federal and state law.
In short, the drugmaker argues that the federal regulations override West Virginia’s abortion restrictions. West Virginia Attorney General Patrick Morrisey, however, plans to defend the abortion ban vigorously because “the U.S. Supreme Court has made it clear that regulating abortion is a state issue.”
In another pending lawsuit, Bryant v. Stein, obstetrician-gynecologist Amy Bryant sued officials in North Carolina on similar grounds. Although North Carolina has not banned abortion, it imposes a number of restrictions, including a 72-hour waiting period before accessing medical or surgical abortions.
Bryant argues that the restrictions exceed the FDA requirements for dispensing mifepristone and are therefore preempted by federal law.
There is limited precedent in this area.
In one case, the manufacturer of an opioid – Zohydro – challenged a Massachusetts ban of the drug, even though the FDA had approved it. The federal court ruled for the manufacturer because the ban would “obstruct the FDA’s Congressionally-given charge.”
That 2014 opinion might suggest that GenBioPro will succeed. On the other hand, a court might distinguish the two cases: Massachusetts banned Zohydro on public health grounds, which is squarely within the FDA’s authority, while West Virginia bans abortions on moral grounds – to protect fetal life – which is outside the FDA’s purview.
In the North Carolina case, the state does not ban mifepristone; it just imposes more restrictions than the FDA requires. Therefore, it is uncertain whether the Zohydro reasoning would be adopted.
A 2008 Supreme Court case, however, might be relevant.
In Wyeth v. Levine, a drugmaker claimed that FDA labeling requirements for a drug made by Wyeth, which was used to prevent allergies and motion sickness, preempted Vermont’s stricter labeling requirements. The Supreme Court rejected that argument. It concluded instead that allowing states to require stronger warnings didn’t interfere with Congress’ purpose in entrusting the FDA with drug labeling decisions.
Wyeth is not precisely like Bryant, however.
Whereas Wyeth dealt with labeling requirements, Bryant deals with regulations that affect access to a drug. Nevertheless, the Wyeth precedent could allow a court to permit states to impose stronger restrictions on access to mifepristone – as long as they fall short of banning the drug outright.
Banning mifepristone
Another pending lawsuit may threaten the FDA’s authority to authorize any sales of mifepristone in the United States.
In Alliance for Hippocratic Medicine v. FDA, a group of abortion opponents asked a U.S. district court in November 2022 to force the FDA to stop allowing mifepristone sales anywhere in the United States. The lawsuit argues that the FDA “chose politics over science” and “exceeded its regulatory authority” in various ways, including allegedly disregarding “substantial evidence” that medication abortion is riskier than surgical abortions.
The consequences could be quite significant, and the issue could even end up on the Supreme Court’s docket in the future. Nevertheless, there are compelling legal reasons why this lawsuit should fail.
Some of the same organizations have tried to challenge the FDA’s approval of mifepristone before – without success. And in 2008, the Government Accountability Office found no irregularities in the FDA’s approval and oversight of mifepristone.
In contradiction to the plaintiffs’ safety argument, numerous studies have shown mifepristone to be a safe and effective drug.
Nevertheless, U.S. District Court Judge Matthew Kacsmaryk, who sits in Amarillo, Texas, and is hearing this case regarding whether the FDA should rescind its approval of mifepristone, has not been supportive of reproductive rights in the past. Thus, it is possible that the court could try to stop the FDA from allowing mifepristone to be sold in that part of Texas or even, possibly, across the entire nation.
If the court prevents the sale of mifepristone nationwide, medication abortions would only be possible with the other pill, misoprostol, which is also approved for other purposes. Recent data suggests that this one-drug approach to medication abortions may safely and effectively induce abortion.
Pills in interstate commerce
In addition to questions of whether the FDA’s authority can override state-imposed abortion restrictions, there’s a second issue concerning the ability to sell the pills through interstate commerce.
As the Supreme Court has explained, the Constitution grants Congress the authority to regulate “things in interstate commerce,” as well as “those activities that substantially affect interstate commerce.”
Thus, in the GenBioPrio lawsuit pending in West Virginia, the company argues that state efforts to restrict sale of the pill are unconstitutional.

Mailing abortion pills
Many people are also taking abortion pills they get through the mail. In response to that trend, 20 Republican state attorneys general recently threatened pharmacies with “legal consequences” if they mail and distribute mifepristone.
An 1873 law, the Comstock Act, is central to the issue of whether it is legal to mail abortion pills. That law makes it a crime to use the mail for any “lewd, lascivious, indecent, filthy or vile article” as well as any “article, instrument, substance, drug, medicine or thing which is advertised or described in a manner calculated to lead another to use of apply it for producing abortion.”
When the Comstock Act was enacted, of course, modern delivery services like FedEx and UPS did not exist. But the law also prohibits any “express company” from engaging in the same acts.
The U.S. Postal Service asked the Justice Department whether abortion pills can be mailed under the Comstock Act, and it responded with a carefully worded 21-page opinion in late December 2022. The opinion concludes that mailing the abortion pills is not illegal so long as the sender “lacks the intent that the recipient of the drugs will use them unlawfully.”
As the opinion pointed out, recipients could use the drugs for a variety of reasons that would be legal in every state. For example, the combination can “treat a miscarriage,” and misoprostol can prevent and treat gastric ulcers.
Regardless of how Judge Kacsmaryk rules, we expect to see medication abortion remain available in states that don’t have abortion bans. But we also are certain that legal challenges over abortion access will continue.
The authors do not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.
Read These Next
Fewer new moms are dying in Colorado – naloxone might be one reason why
The opioid reversal drug is distributed directly to new moms at many of Colorado’s birthing hospitals.
Minneapolis united when federal immigration operations surged – reflecting a long tradition of mutua
Minnesotans from all walks of life, including suburban moms, veterans and protest novices, have bucked …
It’s never too late to learn a language – adults and kids bring different strengths to the task
While a young language learner can more easily acquire a native accent, adults retain the ability to…